Method for L-threonine production
A threonine, microorganism technology, applied in microorganism-based methods, biochemical equipment and methods, microorganisms, etc., can solve problems such as gene expression of threonine operon that are difficult to predict
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
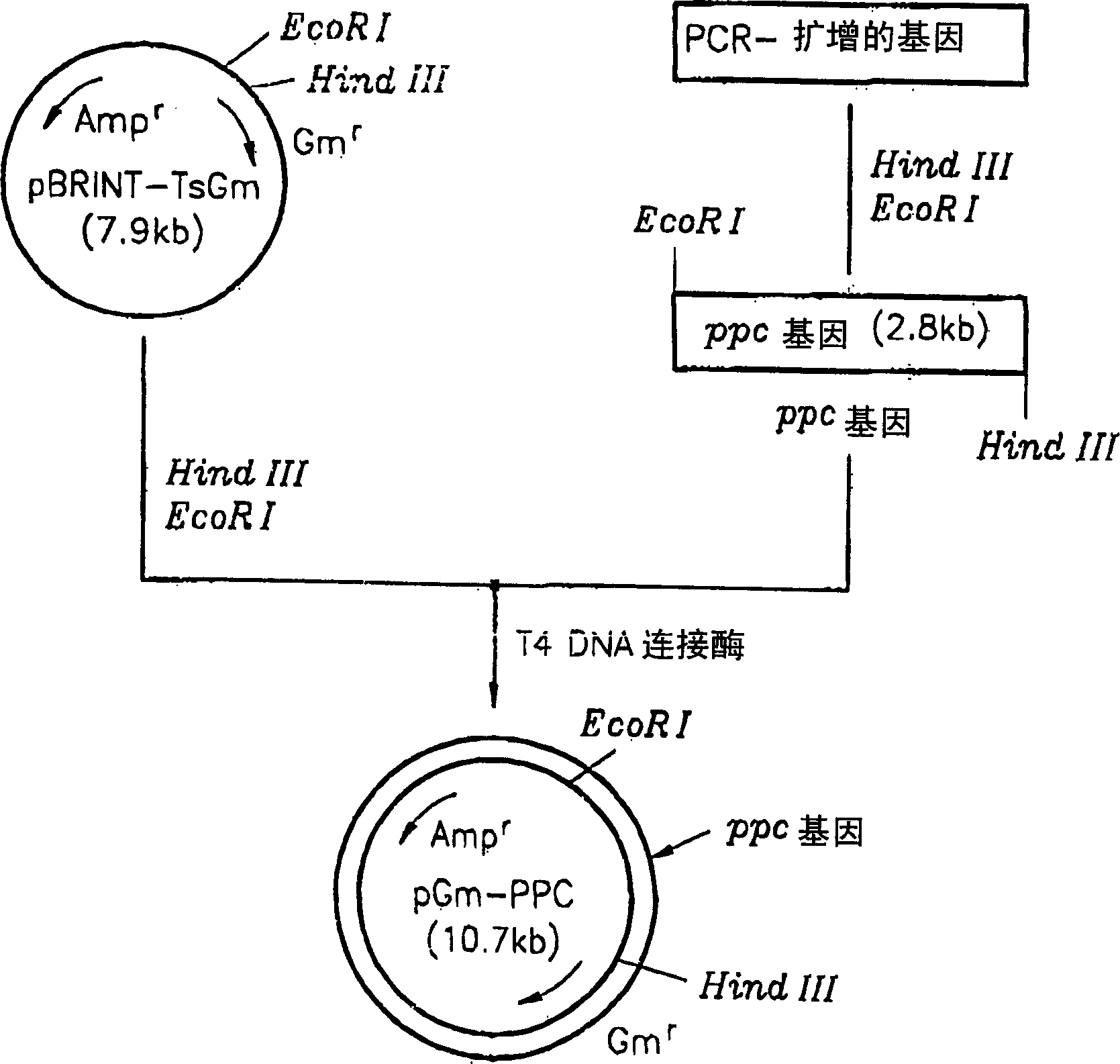
[0022] A method for cloning the phosphoenolpyruvate carboxylase (ppc) gene is described in figure 1middle. The ppc gene was obtained from a threonine producing strain-TF4076. Chromosomal DNA was isolated, digested with restriction enzyme Sal I, and electrophoresed to selectively separate 4-5 Kb DNA fragments. Using the isolated DNA fragment as a template, the ppc gene was amplified using primer 1 (5'-aggaattcttccgcagcatttgacgtcac-3') and primer 2 (5'-aggaagcttttagccggtattacgcatacc-3'). The amplified product was digested with EcoRI and HindIII, and then electrophoresed again to finally isolate a 2.8 Kb ppc gene fragment. Cloned with a 7.6Kb pBRINT-TsGm (a pBRINT-Ts vector) from the National University of Mexico (Sylvie Le Beatriz et al., 1998, pBRINT-Ts: a family of plasmids with temperature-sensitive replicons, for designed for chromosomal integration into the lacZ gene of E. coli, Genes, 223, pp213-219). pBRINT-TsGm was double digested with the same restriction enzymes Ec...
Embodiment 2
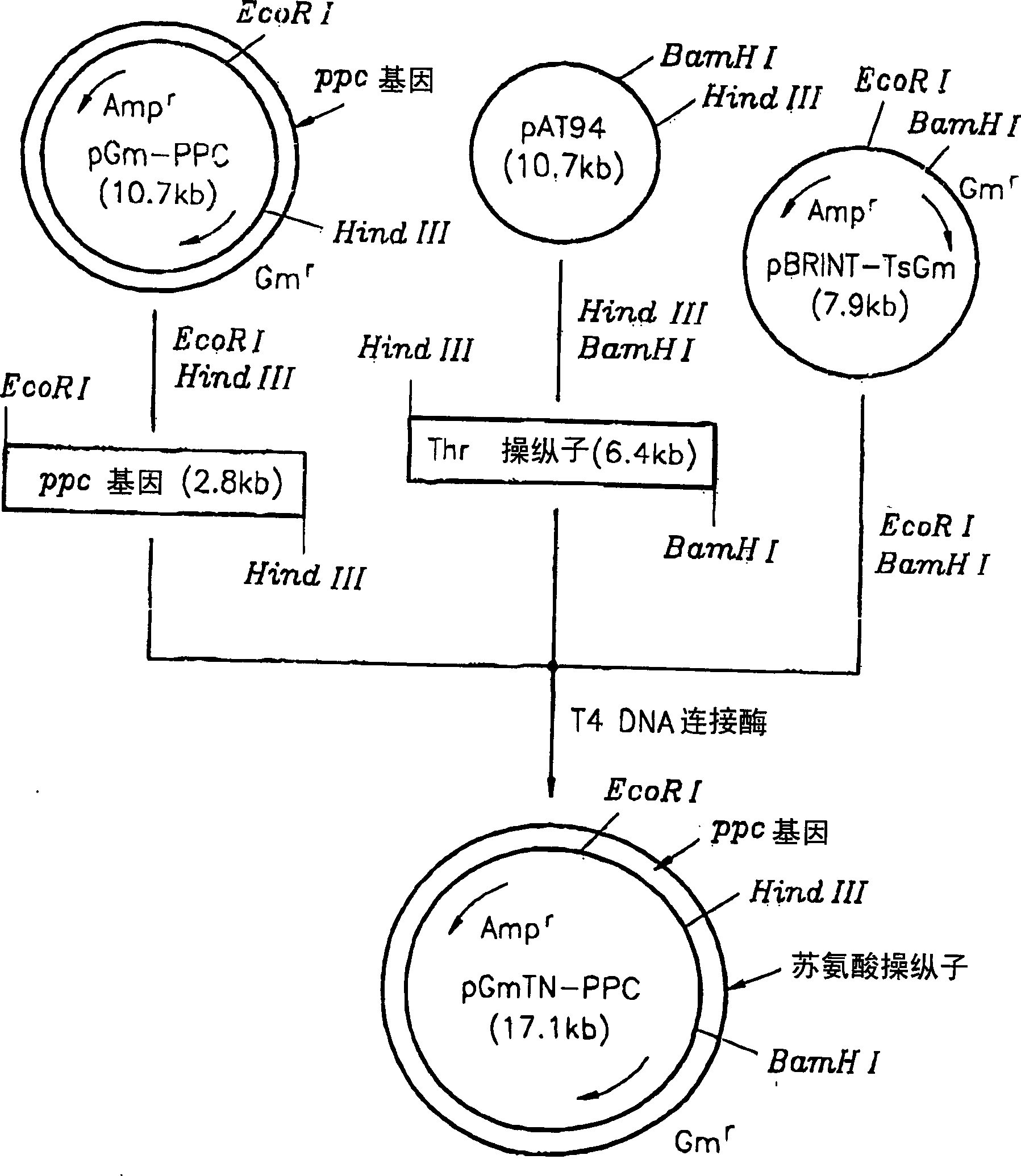
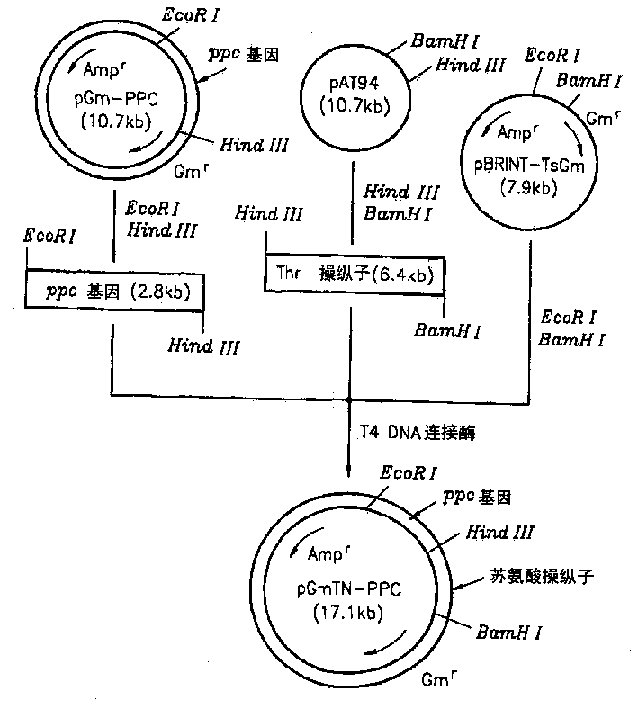
[0023] The recombinant plasmid vector pAT94 (Korean Patent Application No. 92-24732) constructed by cloning chromosomal DNA of TF4076 was used for the threonine operon, and the recombinant plasmid pGmPPC in Example 1 was used for the ppc gene. pBRINT-TsGm (a pBRINT-Ts vector) from the National University of Mexico was used as a chromosomal DNA integration vector (Sylvie Le Beatriz et al., 1998, pBRINT-Ts: a family of plasmids with temperature-sensitive replicons for chromosomal integration into the lacZ gene of Escherichia coli., Gene., pp213-219). figure 2 The construction method of the recombinant plasmid is described. pAT94 was double digested with restriction enzymes HindIII and BamHI. The 6.4 kbp threonine operon DNA fragment was isolated from the double digest by electrophoresis. pGmPPC was double digested with HindIII and EcoRI to isolate the 2.8 kilobase pair ppc gene fragment. The pBRINT-TsGm plasmid vector was digested with EcoRI and BamHI, and the completely dig...
Embodiment 3
[0024] Transform TF4076 (a threonine-producing strain) with the recombinant plasmid pGmTN-PPC isolated from Escherichia coli strain DH5α, in LB solid medium containing 5 mg / L gentamicin (yeast extract 5 g / 10 grams / liter of tryptone for bacteria, 10 grams / liter of sodium chloride; 1.7% of agar for bacterial culture; pH7.0), cultivated at 30 degrees Celsius for 60 hours. Each single colony was inoculated into 0.5 ml of LB and incubated at 30°C for 4 hours. An aliquot of the culture was transferred to 10 mL of LB and incubated at 30°C for 6 hours and then at 37°C overnight. 10 of the culture -3 -10 -6 Dilutions were inoculated into LB solid medium containing 5 mg / L gentamicin. At this time, 12 microliters of IPTG (0.1 M) and 60 microliters of X-gal (2%) were also inoculated on the LB solid medium. Cultivate at 44 degrees centigrade for 24 hours, and screen for recombinant strains that are sensitive to carbenicillin and appear as white colonies, which cannot grow on LB solid ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com