Methods of charging lithium-sulfur batteries
A battery charging and battery voltage technology, applied in battery electrodes, secondary battery charging/discharging, battery circuit devices, etc., can solve the problems of reduced cathode performance and short cycle life, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] The battery was made by the following method.
[0104] Dispersed in isopropanol were 75 parts elemental sulfur (available from Aldrich Chemical Company, Milwaukee, Wisconsin), 15 parts conductive carbon pigment PRINTEX XE-2 (trade name for carbon pigments, available from Degussa Corporation, Akron, Ohio) and 10 parts. A mixture of parts PYROGRAF-III (trade name for carbon fiber filaments, available from Applied Sciences Inc., Cedarville, Ohio) was applied to an aluminum foil substrate (Product No. 60303, available from South, MA) that had been coated with 17 micron thick conductive carbon. Hadley's Rexam Graphics), made the cathode. After drying and calendering, the applied cathode active layer was about 27 microns thick. The anode is a lithium foil about 50 microns thick. The electrolyte was lithium bis(trifluoromethylsulfonyl)imide (commercially available from 3M, St. Paul, Minnesota) in a 50:50 volume ratio of 1,3-dioxolane to dimethylformaldehyde 0.75M solution i...
Embodiment 2-12 and comparative example 1-7
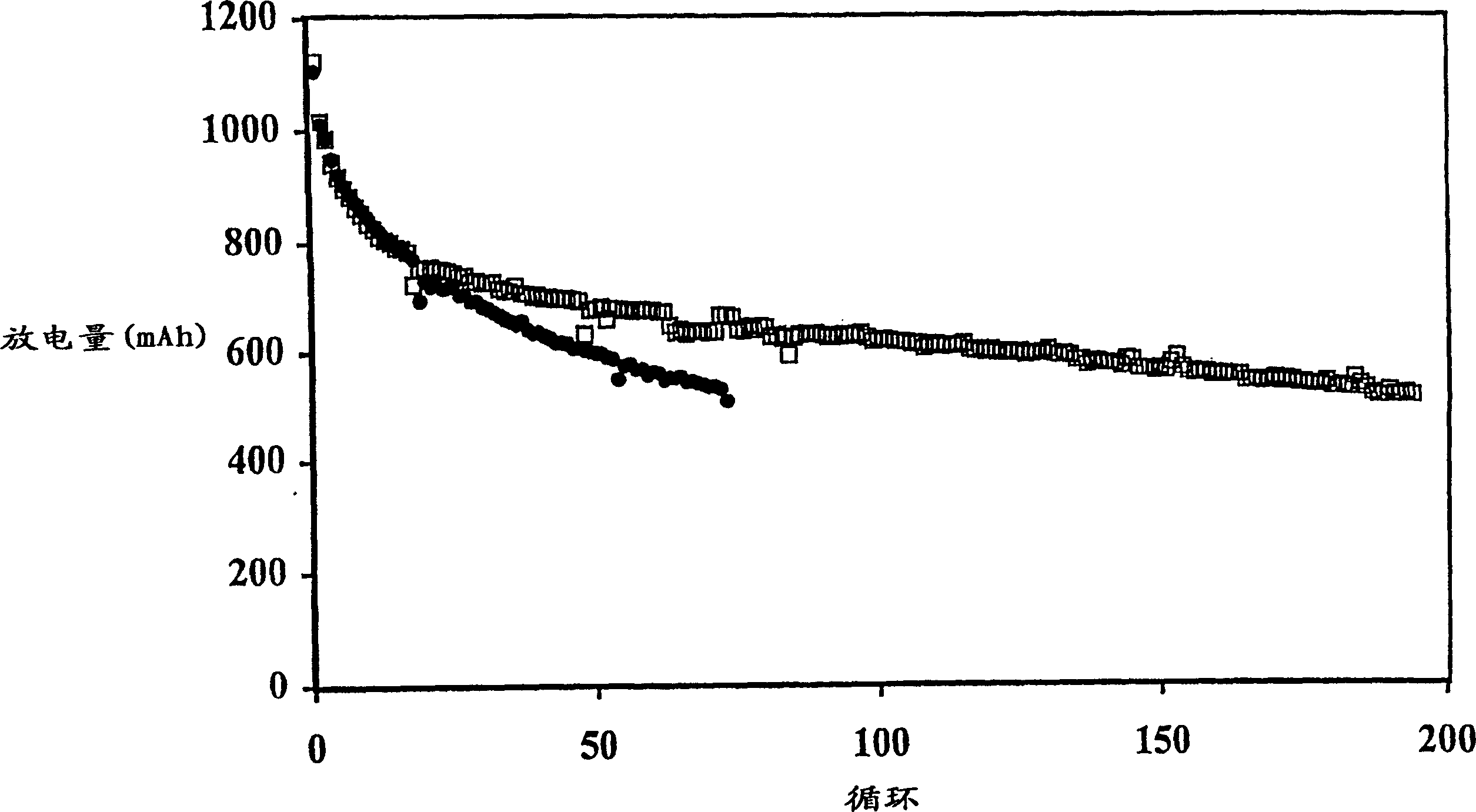
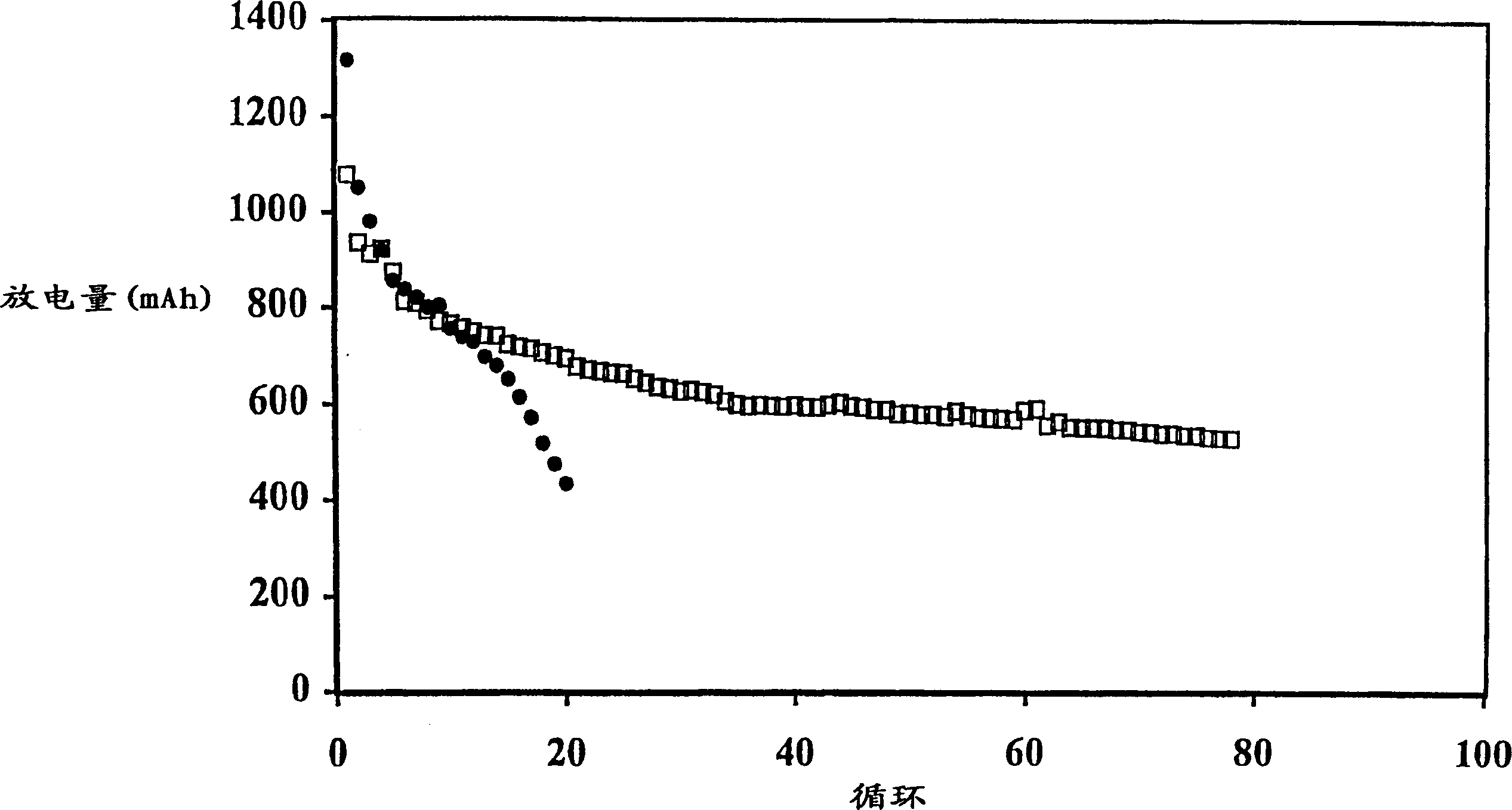
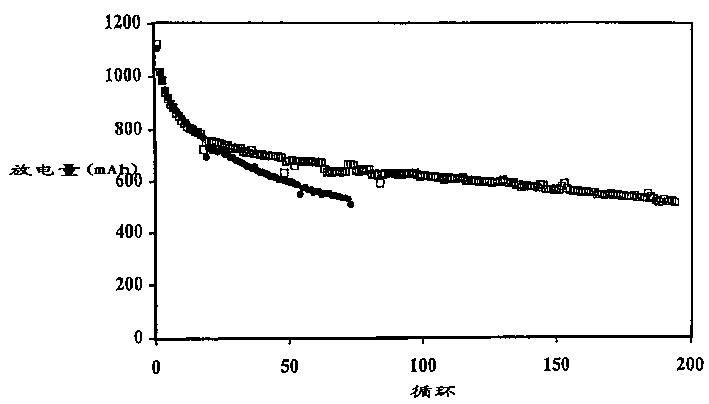
[0107] The battery produced in Example 1 was discharged to 1.5V under the condition of a discharge current of 500mA, 150mA or 50mA. The initial discharge capacity is generally about 1100mAh, and the discharge capacity of the 5th cycle is about 800-850mAh. The results of the batteries charged with the multi-step method of the present invention are shown in Table 2, and the results of the comparative batteries charged with a single step are shown in Table 1.
[0108] In Comparative Example 1, the battery was charged by the current density decreasing charging method, in which the battery was charged at a high current of 200 mA (0.25 mA / cm 2 ) to a voltage of 2.3V and then held at that voltage for 5 hours, during which time the charge current was reduced to a low level, eg 50mA.
[0109] Table 1
Relationship between battery performance and discharge-charge conditions
Comparative scale number
Discharge current
recharging curren...
Embodiment 13
[0113] table 3
[0114] The data in Table 3 illustrate that the multi-step charging method of the present invention results in less swelling of the prismatic cells. For example, Comparative Example 6 exhibits an undesirably larger overall expansion rate at 500 mAh cutoff compared to Example 2. Comparing the expansion (or FOM) per cycle, the advantages of the two-step approach are more apparent.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com