Method for producing antithrombotic medicine by using silk-worm
An antithrombotic drug and the technology of silkworm, which is applied in the direction of drug combination, pharmaceutical formula, and the use of carriers to introduce foreign genetic material, etc., can solve the problems of product inhomogeneity, cumbersome chemical method operation, etc., and achieve good effect and high clinical application value.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014]Take Chinese kidney cells, grind them at low temperature, add 1ml of Trizol RNA extraction solution produced by GIBCOBRL company, shake gently for 10 minutes, add 500μl of chloroform (Zhejiang Dier Pharmaceutical Co., Ltd.), place at room temperature for 10 minutes, and centrifuge at 12000rpm for 10 minutes. Minutes, take the supernatant, add 2 times the volume of ethanol, after mixing, centrifuge at 12000rpm for 10 minutes, discard the supernatant, add 1000 units of reverse transcriptase (GIBCOBRL company) and 4dNTP (GIBCOBRL company) for reverse transcription, 37 ℃ , 1 hour to obtain cDNA synthesized by reverse transcription of mRNA. Design primers according to the published urokinase gene (Nagai et al., Gene, 1985, 36:183), we designed two pairs of primers P1 and P2: P1: 5'GGGGATCCATGTTAAAATTTCAGTGTGGCAAAAGAC3'P2: 5'GGGCTCGAGATATTTCTTCTGGAATTTCTTCGAAATCGCCGAGGGCCAGGCCATTCTC3', using cDNA as a template, PCR amplification was carried out with P1 and P2 as primers. The ...
Embodiment 2
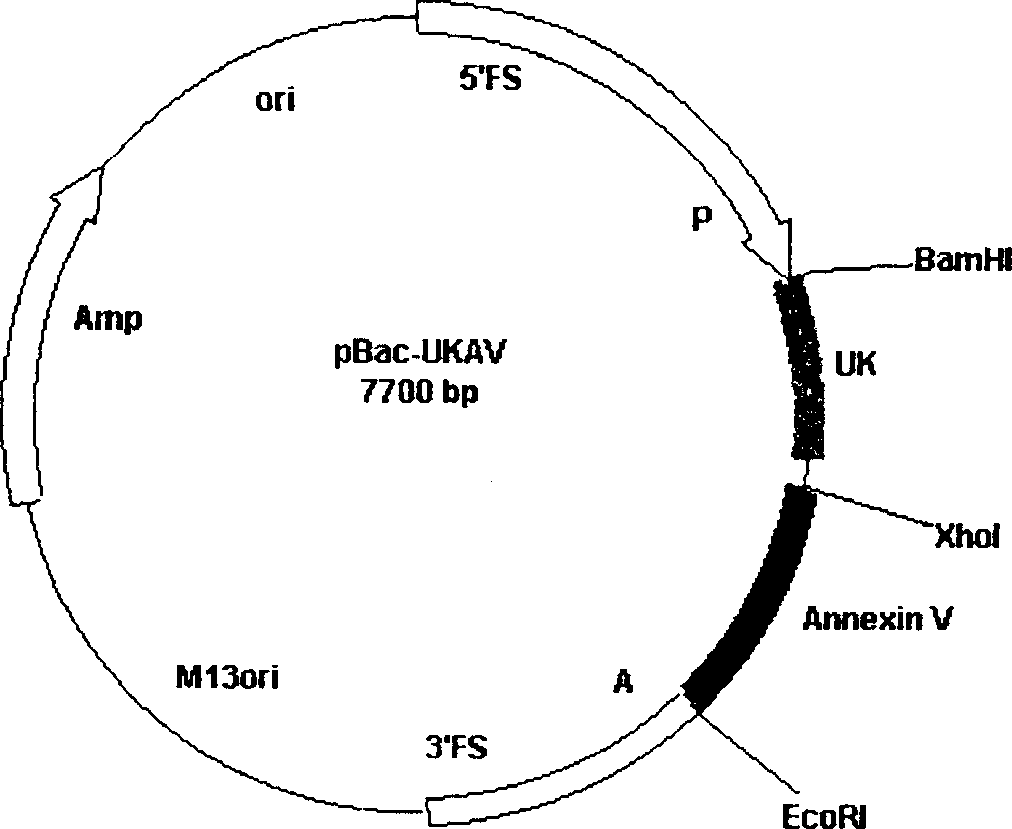
[0015] The plasmid pUC-UKAV was first digested with BamHI and EcoRI, and cloned into pBaePAK8 (CLONTECH Company) digested with BamHI and EcoRI (GIBCOBRL Company), to construct the fusion gene transfer plasmid pBac-UKAV containing AnnexinV and low molecular weight urokinase. All identification is correct. (Fig. 2) Example 3, the acquisition of recombinant insect baculovirus containing Annexin V and low molecular weight urokinase fusion gene
Embodiment 3
[0016] Take 5ul insect baculovirus transfer plasmid pBac-UKAV containing Annexin V and low molecular weight urokinase fusion gene and 6ul silkworm wild nuclear polyhedrosis virus DNA for co-transfection. Take 6ul Lipofectin (GIBCOBRL company) and add 100ul serum-free TC-100 medium and mix well. The BmN cells previously cultured in a 35mm Dish were washed twice with serum-free TC-100 (GIBCOBRL company) medium, and the transfer plasmid and Lipofectin mixture was added dropwise, cultured at 27°C for 4-5 days, and the supernatant was collected for the second stage. One round of plaque screening. Take 5ul of the supernatant to infect the BmN cells in a 35mm Dish, discard the supernatant after 1 hour and add an equal amount of mixed TC-100 medium and low melting point agarose. Pick plaques after 4-5 days, infect BmN cells for 3-4 days, save the supernatant, lyse the cells with NaOH for Southern hybridization, and take the supernatant of positive clones for the 10th round of plaque ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com