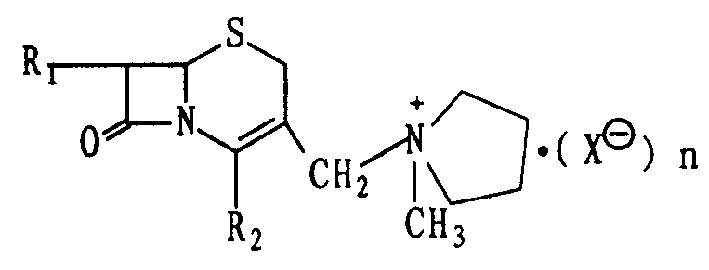
Cephalo olefine onium salt compound and its preparing method, and method for synthesizing cephalo pyoxime with said compound
A technology of cephems and compounds, which is applied in the field of synthesizing cefepime hydrochloride, can solve problems such as high cost, and achieve the effects of simple reaction route, reduced production cost, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] The present invention will be further described below in conjunction with embodiment:
[0025] 1. Preparation of 7β-formamido-3-chloromethyl-3-cephem-4-carboxylic acid p-methoxybenzyl ester (III):
[0026] Take 60g of 7β-amino-3-chloromethyl-3-cephem-4-carboxylic acid p-methoxybenzyl ester hydrochloride (II) and 120ml of tetrahydrofuran, add them to a 250ml reaction flask in turn, and drop them under ice bath Add 20.5ml of triethylamine, after dropping, remove the ice bath, stir at room temperature for 1 hour, filter, wash with 60ml of tetrahydrofuran three times, and set the filtrate for use.
[0027] In another 250ml reaction bottle, add 27.3ml of formic acid and 68.4ml of acetic anhydride, stir and react at 40-50°C for 1 hour, filter, pour the above-mentioned filtrate into it, and precipitate light yellow crystals, continue to react at 45°C and keep warm After 1 hour, cool down, stir in an ice bath for 1 hour, filter, and wash with ethanol to obtain 38.1 g of white ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com