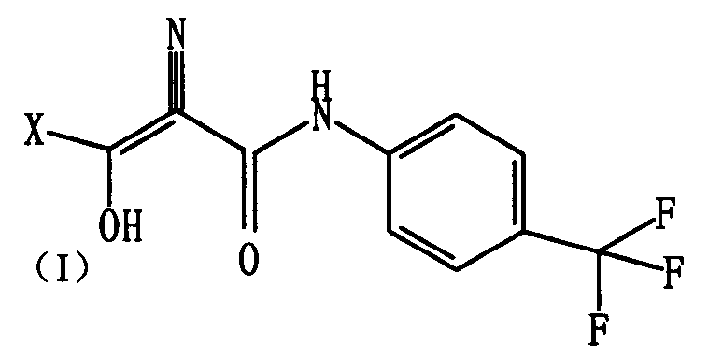
Medicinal composition for treating autoimmune disease
An autoimmune and compositional technology, applied in the field of autoimmune disease treatment, can solve problems such as adverse reactions, achieve the effect of alleviating pathological manifestations, obviously relieving effect, and controlling deposition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The synthesis of embodiment 1 fluosemide
[0028] Take 10 g (0.037 mol) of leflunomide, add it into a flask containing 240 ml of 2% sodium hydroxide solution, and stir at 23-25° C. for 1 hour. Then add 1300ml of water to the reaction solution, filter out the insoluble matter, add 26ml of concentrated hydrochloric acid to the colorless filtrate, immediately obtain a white precipitate, and the pH of the filtrate at this time=1-2. The white solid was collected by filtration, washed with water (3×40ml), and concentrated in vacuo at 40°C to obtain about 10g of the crude product.
[0029] The crude product was recrystallized with 180 ml of absolute ethanol / ethyl acetate (1:8). The precipitated crystals were vacuum-dried at 40°C to obtain 8 g of needle-like crystals (melting point 230-231.5°C). Thin layer chromatography (with CH 2 Cl 2 / HOAc=3 / 1 is developing agent) and 1 H-NMR was used to detect product purity.
Embodiment 2
[0030] The preparation of embodiment 2 capsules
[0031] Formula (1000 Capsules):
[0032] Fluosemide 1g-1000g
[0033] Starch 2.5g-2500g
[0034] Grind the fluosemide raw material into fine powder and pass through a 100-mesh sieve; dry the starch and pass through a 120-mesh sieve; mix the raw and auxiliary materials by the equal-volume incremental method, and pass through a 120-mesh sieve 2-3 times to fully mix; after passing the inspection Fill the capsule.
Embodiment 3
[0035] The preparation formula of embodiment 3 tablet (1000):
[0036] Fluosemide 1g-1000g
[0037] Lactose 5.0g-5000g
[0038] Starch 1.5g-1500g
[0040] 5% starch slurry appropriate amount
[0041]Low-substituted hydroxypropyl cellulose 0.1g-1000g
[0042] OPADRY-OY-GM7305 Appropriate amount
[0043] WHITE
[0044] Grind the fluosemide raw material into fine powder and pass through a 100-mesh sieve; pass the auxiliary materials lactose, starch, and L-HPC through a 100-mesh; use the equal amount incremental method to mix the raw and auxiliary materials, and pass through a 80-mesh sieve 3-4 times to fully mix; Add starch slurry to the mixture to make soft materials, pass through 24 mesh to make wet granules, and dry them in a ventilated oven at 55-60°C; add talc powder after the dry granules are sized through 20 mesh, and punch them with a shallow concave with a diameter of 6.0mm Tablets are made into cores.
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com