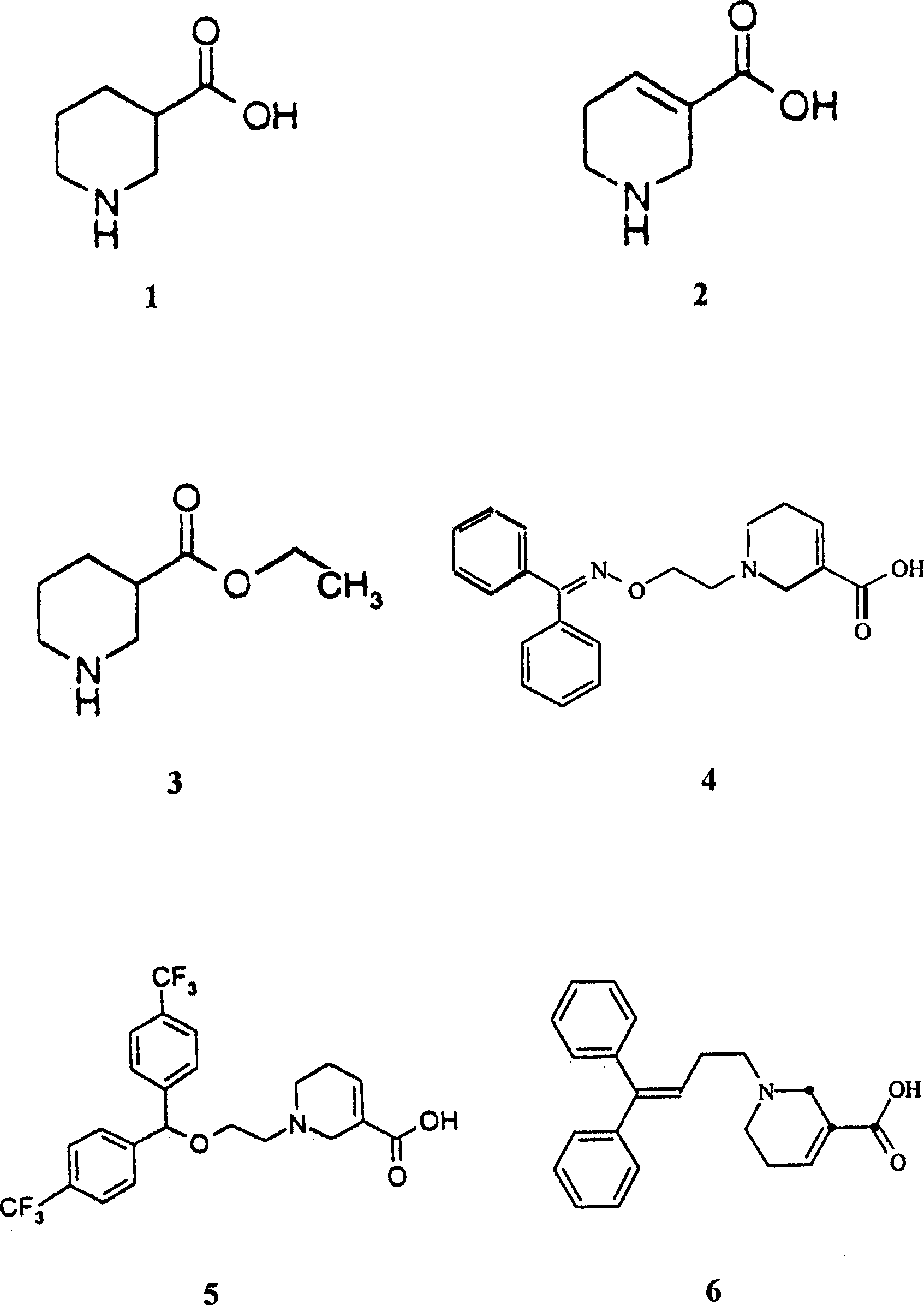
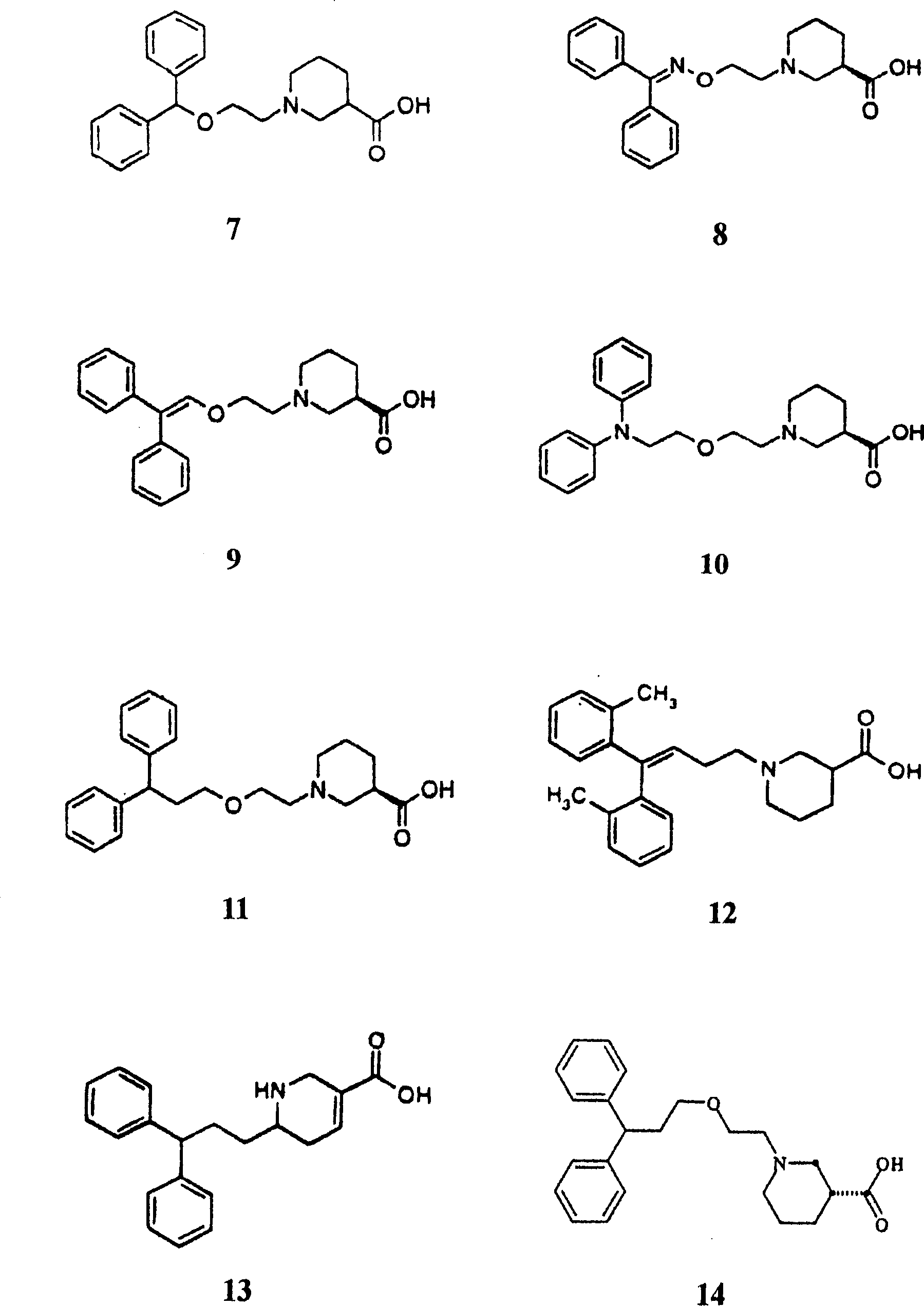
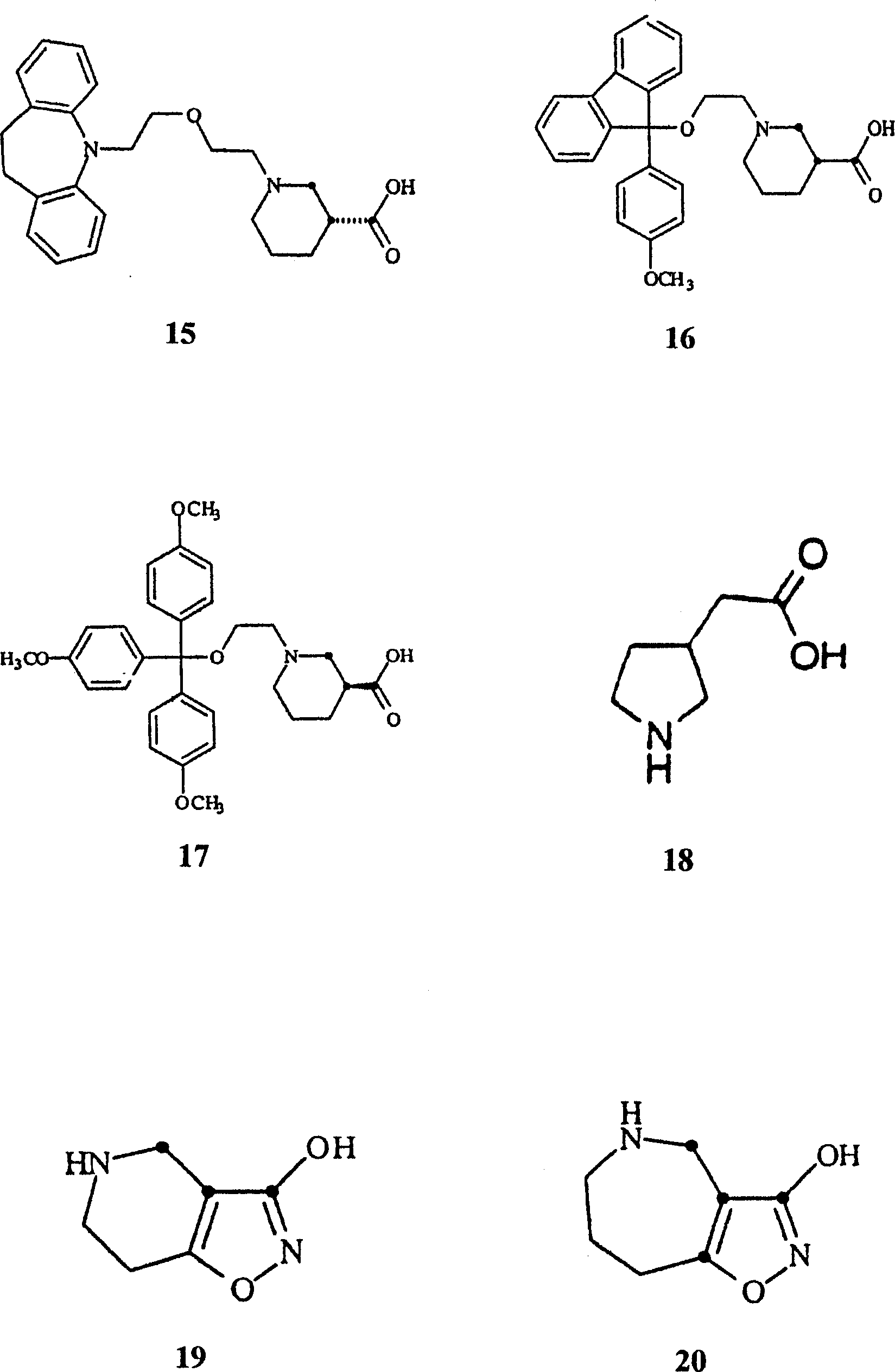
Application of gammalon transporter inhibitor in preparing analgesic
A technology of transporter and aminobutyric acid, which is applied in the direction of drug combinations, pharmaceutical formulas, active ingredients of heterocyclic compounds, etc., can solve problems such as large side effects, achieve non-addictive effects, and avoid dose escalation effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Determination of the analgesic effect of ethyl 3-piperidinecarboxylate and NO-711 on heat-induced pain
[0022] There are 8-10 mice in each group. The tail flick experiment uses 51.0 ℃ hot water to soak the tail. The experimental operation is as follows: (1) Wrap the mouse with a soft cloth, and expose the tail. (2) Immerse the 3 / 4 length of the tail in hot water. To the time interval of tail flicking, (3) mice were administered 3-piperidinecarboxylate ethyl 30mg / kg, 60mg / kg, NO-711 10mg / kg or normal saline of the same volume by intraperitoneal injection respectively, (4) drugs The delay time of tail flick was tested at 5, 15, 30, 45, 60, 75, 90, 105, and 120 minutes after injection, that is, steps (1) and (2) were repeated. To avoid damage to the tail tissue, the maximum immersion time in hot water is 12 seconds. The analgesic effect was calculated by the following formula: %MPE=(delay time after administration-delay time before administration) / (12-delay time before ...
Embodiment 2
[0025] Determination of the analgesic effect of ethyl 3-piperidinecarboxylate and NO-711 on formalin-induced inflammatory pain in the foot
[0026]The experimental operation is as follows: (1) Place the mouse alone, record the time when the mouse licks or bites the left paw, and record it every 5 minutes, and record three time periods, (2) Subcutaneous injection on the back of the left paw of the mouse 10 μl of 5% formalin, immediately record the length of time the mouse licks or bites the injection site, and records it every 5 minutes, (3) after recording the second time interval, that is, 10 minutes after the intraperitoneal injection of 60mg / kg Ethyl 3-piperidinecarboxylate, 10 mg / kg NO-711 or the same volume of normal saline, (4) then record the time of mouse licking or biting the paw, and record it every 5 minutes until 55 minutes after formalin injection. Minutes expire. Significance was assessed with one-way ANOVA. There are 8 mice in each group.
[0027] Measurement...
Embodiment 3
[0029] Determination of the analgesic effect of ethyl 3-piperidinecarboxylate and NO-711 on visceral pain induced by acetic acid
[0030] The experimental operation is as follows: subcutaneously inject 30mg / kg ethyl 3-piperidinecarboxylate, 60mg / kg ethyl 3-piperidinecarboxylate, 5mg / kg NO-711, 10mg / kg NO-711 or the same volume of normal saline (Control group), put back in the mouse cage immediately; (2) Take out the mouse after 5 minutes, inject 0.6% acetic acid (10 μ l / g) in the mouse intraperitoneally, put back in the mouse cage immediately; (3) 5 After 10 minutes, the number of writhing times of the mice was recorded, and the recording was continued for 15 minutes, and the significance was evaluated by one-way ANOVA, with 8-9 mice in each group.
[0031] As a result of the determination, the number of writhing times of mice injected with ethyl 3-piperidinecarboxylate or NO-711 was significantly lower than that of mice in the control group. The response of mice to visceral ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com