Fusion protein possessing growth facilitation action and its coding gene and uses
A technology of fusion protein and action, applied in the direction of peptide/protein components, medical preparations containing active ingredients, hybrid peptides, etc., can solve the problems of long treatment cycle, short half-life of GHRH, unbearable for patients, etc., and achieve broad application prospects , high expression, easy to purify
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Embodiment 1, the acquisition of fusion protein gene HSA-GHRH
[0064] Primers were designed according to the nucleotide sequences of the human serum albumin gene (HSA) and the GHRH protein gene (GHRH), and the human serum albumin gene and the GHRH protein gene were respectively amplified by PCR. The primer sequences were as follows:
[0065] Primer 1: 5'-GGGAATTCCTATTACAATCTAGCTCTAGCACCTCT-3';
[0066] Primer 2: 5'-GTGGTGGTGGTTCCGGTGGTGGTGGTGGTTCTTACGCTGACGCTATCTTCACTAAC-3';
[0067] Primer 3: 5'-ACGCTGACGCTATCTTCACTAACTCCTACCGTAAGGTTTTGGGTCAATTGTCCGCTAGAAAGTTGTTGCAAGACATCATG-3';
[0068] Primer 4: 5'-CAATCTAGCTCTAGCACCTCTCTCTTGGTTGGACTCACCTTGTTGTCTGGACATGATGTCTTGCAACAACTTTCTAGGGGA-3'.
[0069] 1. Amplification of the GHRH gene
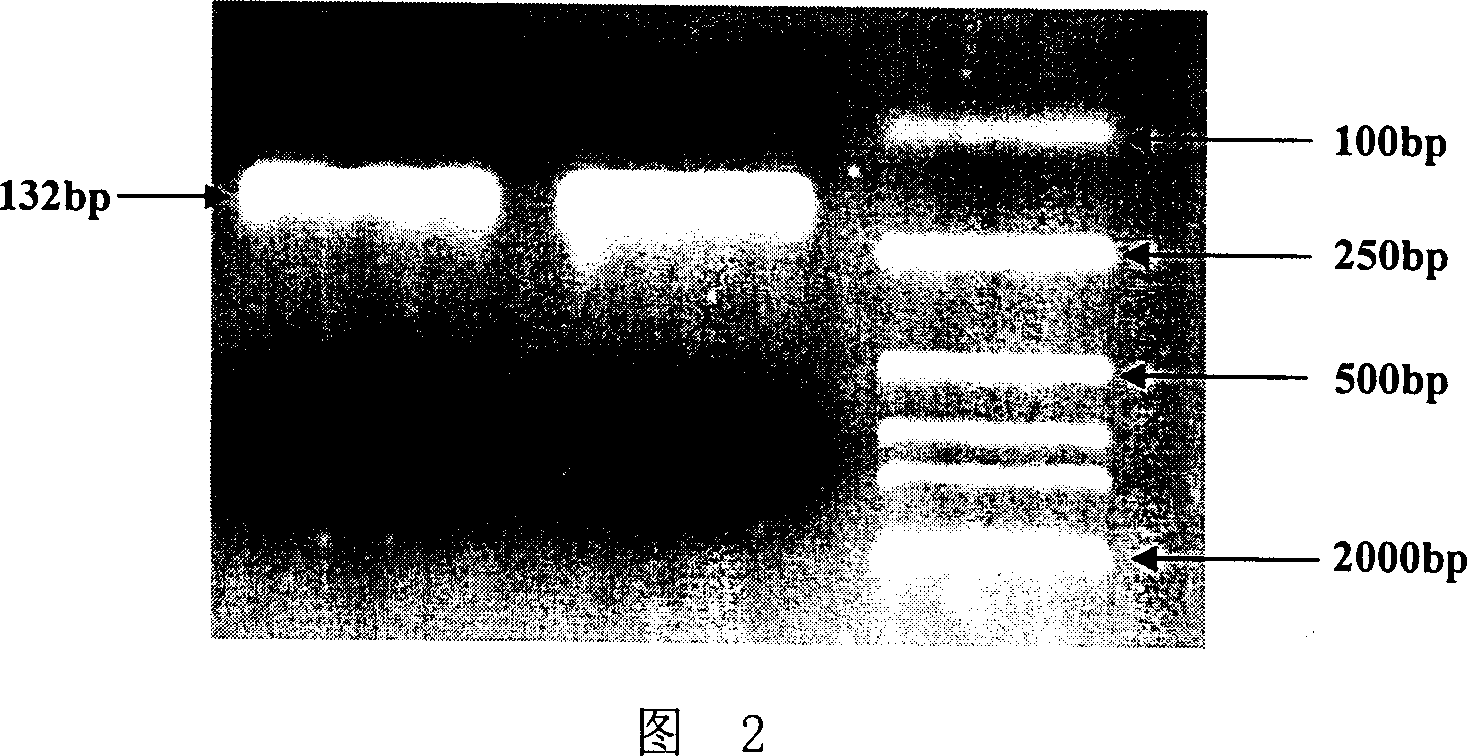
[0070] Using primers 3 and 4 as templates and primers, the full-length sequence of the GHRH gene was assembled by PCR. The 50 μl PCR reaction system was: 5 μl of 10x PCR buffer, 1.5 μl of dNTP, 0.5 μl of Pfu enzyme, primers 3 and 4 2 μl ea...
Embodiment 2
[0076] Example 2, Expression of Human Serum Albumin and Human Growth Hormone Releasing Hormone Fusion Protein Gene HSA-GHRH in Pichia pastoris and Purification of Expression Products
[0077] 1. Expression of human serum albumin and human growth hormone releasing hormone fusion protein gene HSA-GHRH in Pichia pastoris
[0078] 1. Screening of high expression transformants
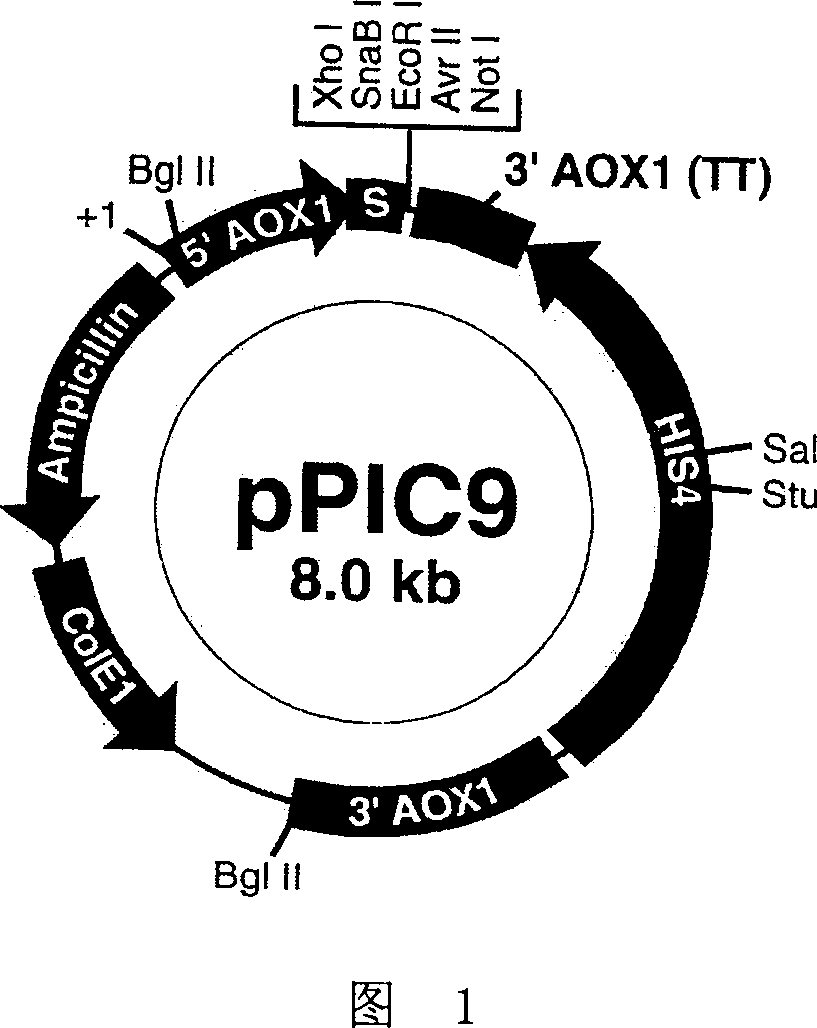
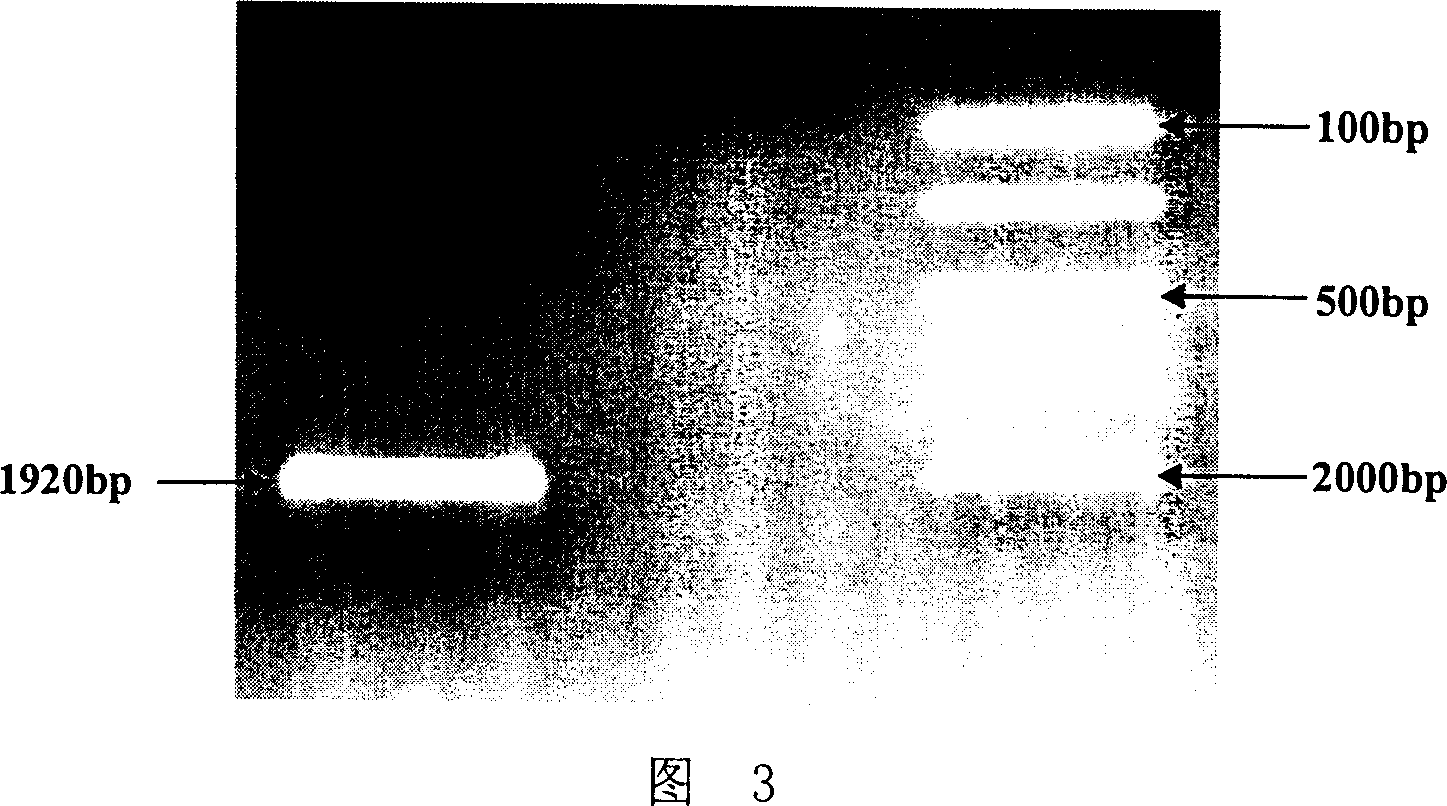
[0079] The recombinant yeast expression vector HSA-GHRH / pPIC9 of HSA-GHRH constructed in Example 1 was transformed into Pichia pastoris GS115 his4(Mut+his-)NRRLY-15851 by the yeast protoplast transformation method, and the specific method was: linear Add about 10 μg of HSA-GHRH / pPIC9 plasmid DNA into 100 μl Pichia pastoris GS115 protoplasts, and let stand at room temperature for ten minutes. Add 1.0 mL of fresh 40% PEG / CaT solution, mix gently and incubate at room temperature for 10 minutes. After centrifugation at 750g for 10 minutes, carefully aspirate the PEG / CaT solution, and resuspend the cells with ...
Embodiment 3
[0084] Embodiment 3, animal experiments
[0085] 1. In vivo activity detection of fusion protein HSA-GHRH in mice
[0086] Twenty 3-week-old weaned male Kunming mice were randomly divided into 4 groups, 5 mice in each group, one group was the control group, and the other 3 groups were the treatment groups. In the treatment group, 0.2 mL of the HSA-GHRH fusion protein obtained in step 2 was subcutaneously injected per day, and the injection volumes in each group were 20, 2, and 0.2 μg; the control group was subcutaneously injected with 0.2 mL of PBS per day. The body weight was recorded daily, and the administration was continued for two weeks. The experimental results are shown in Fig. 10, the group whose daily dosage is 0.2 μg has the most obvious weight gain effect, and the average daily weight gain percentage is 46% higher than that of the control group.
[0087] 2. In vivo long-term detection of fusion protein HSA-GHRH in mice
[0088] Twenty 3-week-old weaned male Kunm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com