Constructing genetic engineering Vaccine of adhesin of confluent Helicobacter pylor and preparation method
A genetically engineered vaccine, Helicobacter pylori technology, applied in the direction of peptide preparation methods, genetic engineering, botany equipment and methods, etc., can solve the complex anti-Hp treatment, can not effectively prevent Hp reinfection and recurrence, single-dose drug treatment low eradication rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
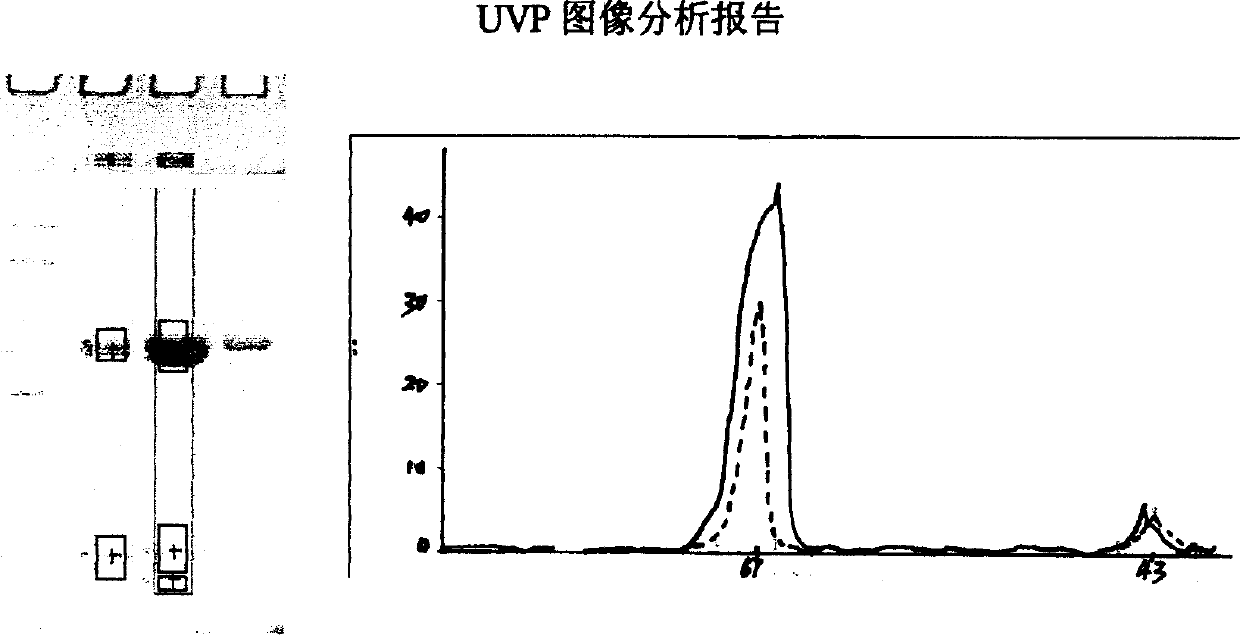
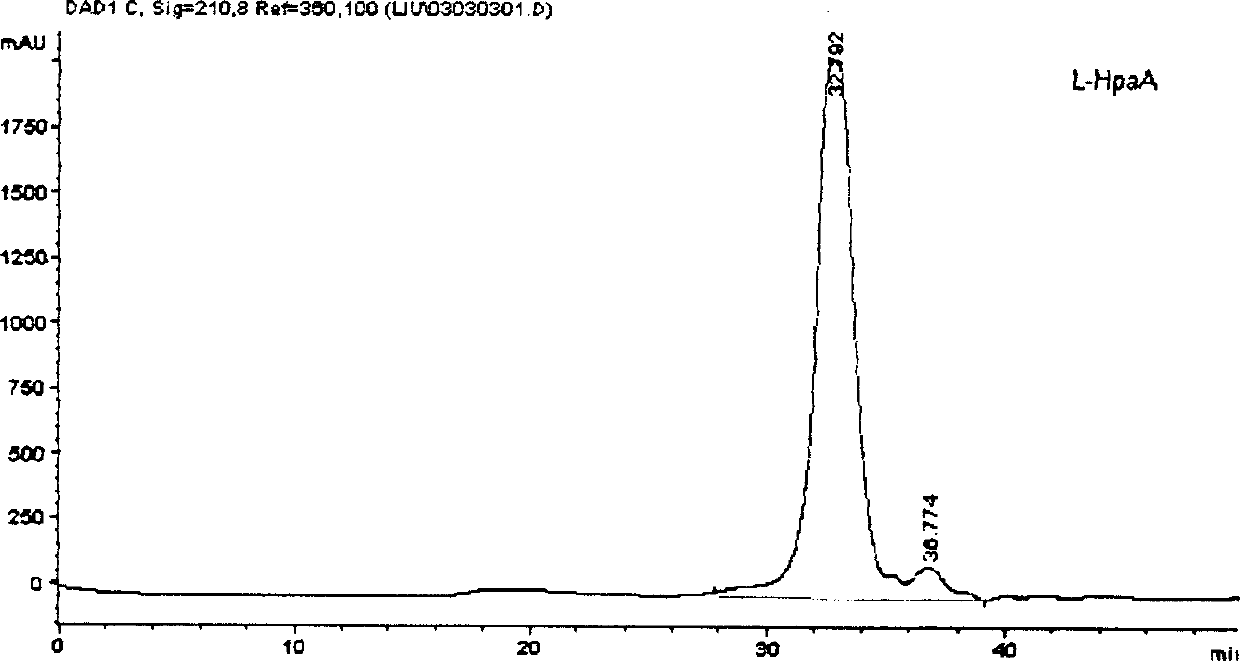
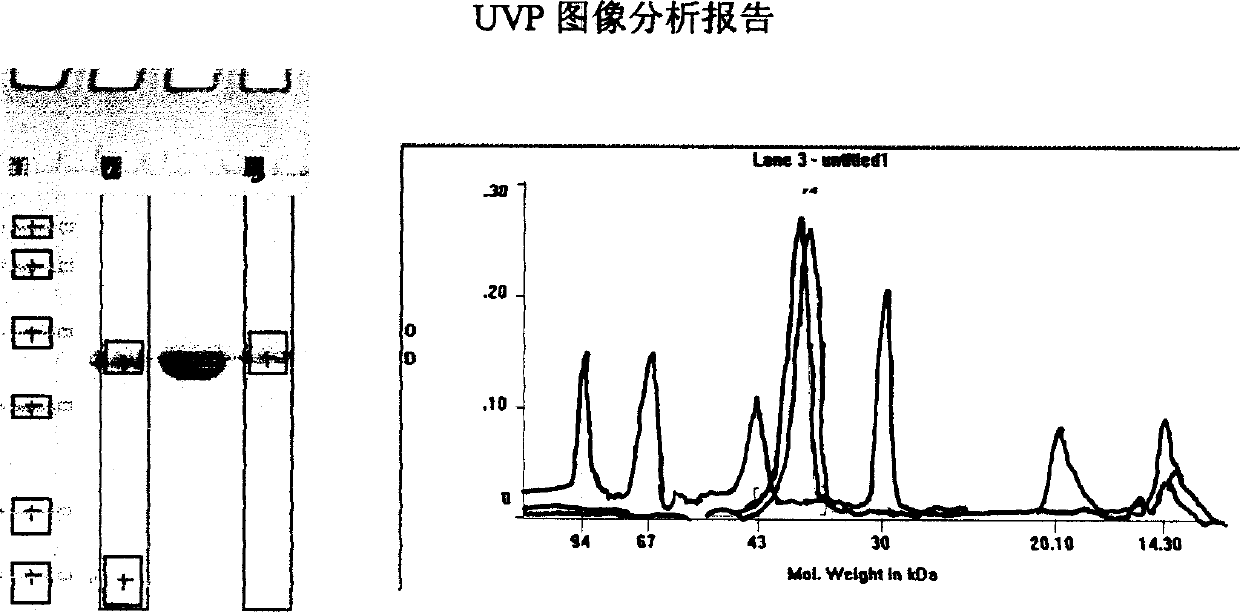
Image
Examples
Embodiment 1
[0045] Embodiment 1, construction and preparation method of LTB / HpaA genetic engineering vaccine
[0046] 1. Construction of genetically engineered bacteria
[0047] 1. Primer design and synthesis
[0048] Using wild-type toxigenic Escherichia coli H44815 (purchased from China National Institute for the Control of Pharmaceutical and Biological Products, stored in our laboratory), Helicobacter pylori SS1 (Hp Sydney strain, named by Lee A in Australia, etc., stored in our laboratory) as templates, use P1 and P2, P5 and P6 to amplify the LTB and HpaA genes, and the bacterial genome was extracted according to conventional methods (Yan Ziying, translated by Wang Hailin. Refined Guide to Molecular Biology Experiments. Science Press, 1998, P39). The PCR amplification system is: 10 μL of amplification buffer without magnesium ions, 10 μL of MgCl 2 (25mmol / L) 10μL, dNTPs (2.5mmol / Leach) 8μL, upstream and downstream primers (P1 and P2 or P5 and P6) 2μL each, the above LTB genome 2μL, ...
Embodiment 2
[0062] Embodiment two, the construction and preparation method of CTB / HpaA genetic engineering vaccine
[0063] 1. Construction of genetically engineered bacteria
[0064] 1. Primer design and synthesis
[0065] Using Escherichia coli 0139 (from the American Type Culture Collection, ATCC31165) and Helicobacter pylori SS1 genomic DNA as templates respectively, CTB and HpaA genes were amplified with P3 and P4, P5 and P6, and the genome extraction method was the same as in Example 1 . The PCR amplification system was: 10 μL of 10× Mg+ ion-free amplification buffer, 10 μL of MgCl2 (25 mmol / L), 8 μL of dNTPs (2.5 mmol / L each), 2 μL of upstream and downstream primers (P1 and P2), and the above CTB genome 2 μL, Ex-Taq DNA polymerase (3 units / μL) 1 μL, add sterilized water to 100 μL.
[0066] PCR amplification reaction: pre-denaturation at 94°C for 5 min, denaturation at 94°C for 50 s, annealing at 60°C for 50 s, extension at 72°C for 50 s, 35 cycles, and complete extension at 72°C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com