Stable liquid interferon formulations
A technology of interferon and stabilizer, which is applied in the field of β-interferon liquid preparations, can solve the problems of increasing needle-stick injuries and dripping ingredients, and achieve the effects of reducing drug delivery methods, improving dosage accuracy, and simplifying packaging
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 3
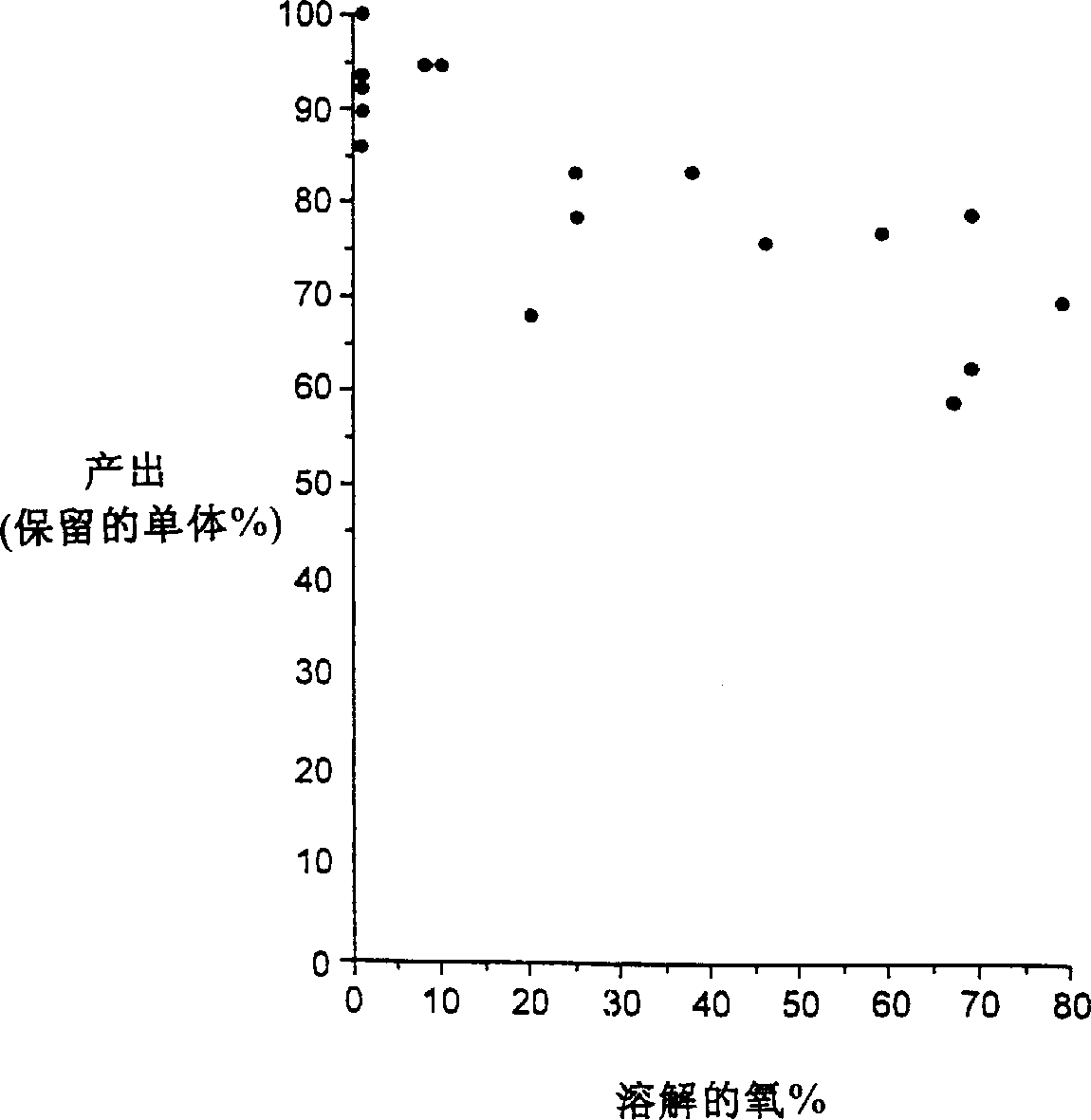
[0072] 5. Adsorption of interferon to the surface
[0073] We have also determined that interferon will adsorb to certain surfaces, and its storage in glass containers requires that at least one surface of the container in contact with the interferon be coated or covered with a material that prevents or substantially eliminates adsorption. The surface may be chemically or physically inert to adsorption. Exemplary materials for this purpose are known to those of ordinary skill in the art and may include, for example, sprayed or baked silicone, polypropylene or polytetrafluoroethylene (PTFE). We used the preferred 60 μg / ml liquid formulations (BG9589-1, 2, 3 and 4: summarized in Table 1 below), filled in 1 ml long Type I glass syringes and 0.75 ml Type I glass vials, which Spray-on silicone (Beckon Dickinson) was applied. Samples were then analyzed by reverse phase HPLC (rpHPLC) to determine protein concentration. The data showed that sample solutions filled in glass vials ha...
Embodiment 8
[0117] The following examples are provided to illustrate embodiments of the invention, but should not be construed as limiting the scope of the invention.
Embodiment 1
[0118] Embodiment 1: test method
[0119] The physicochemical properties of beta-interferon in our liquid formulations were determined using several well-characterized methods, which can also be used to measure the properties of other interferons.
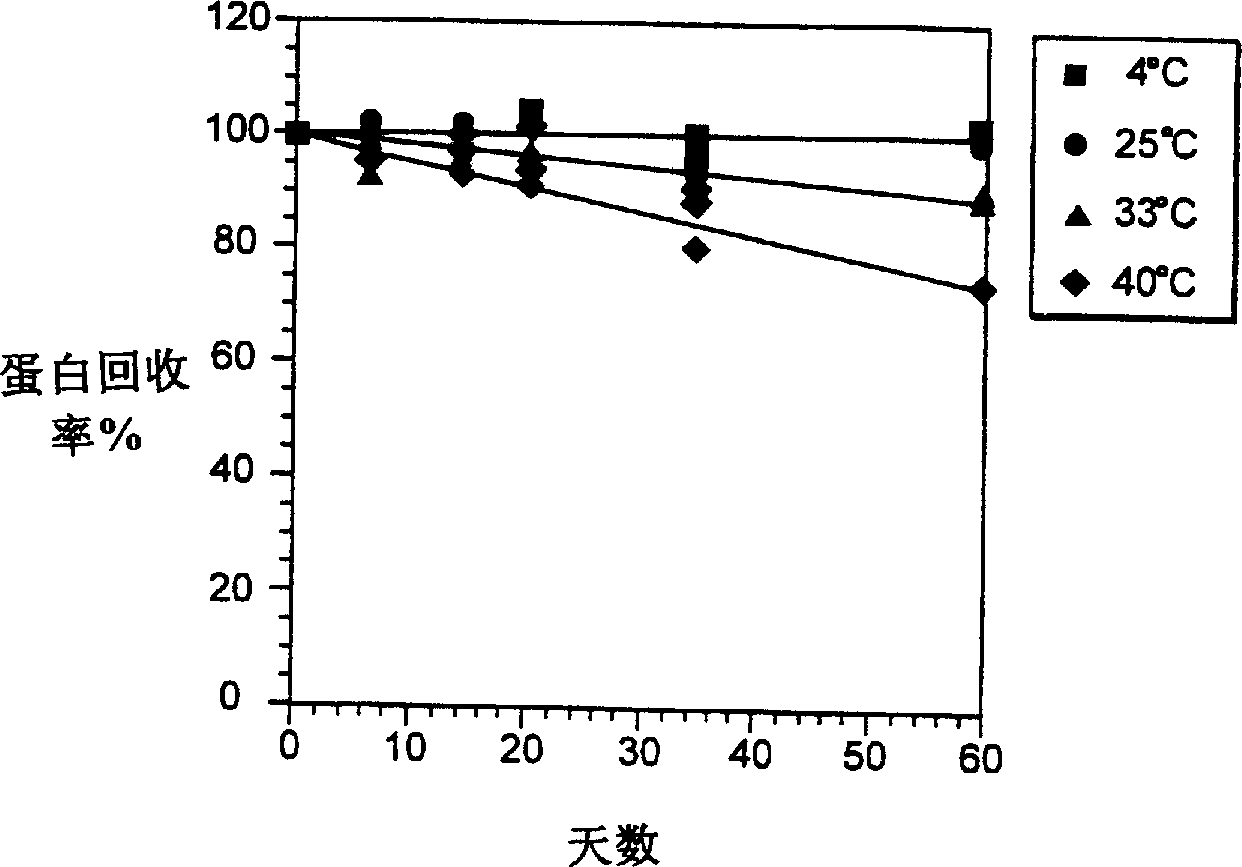
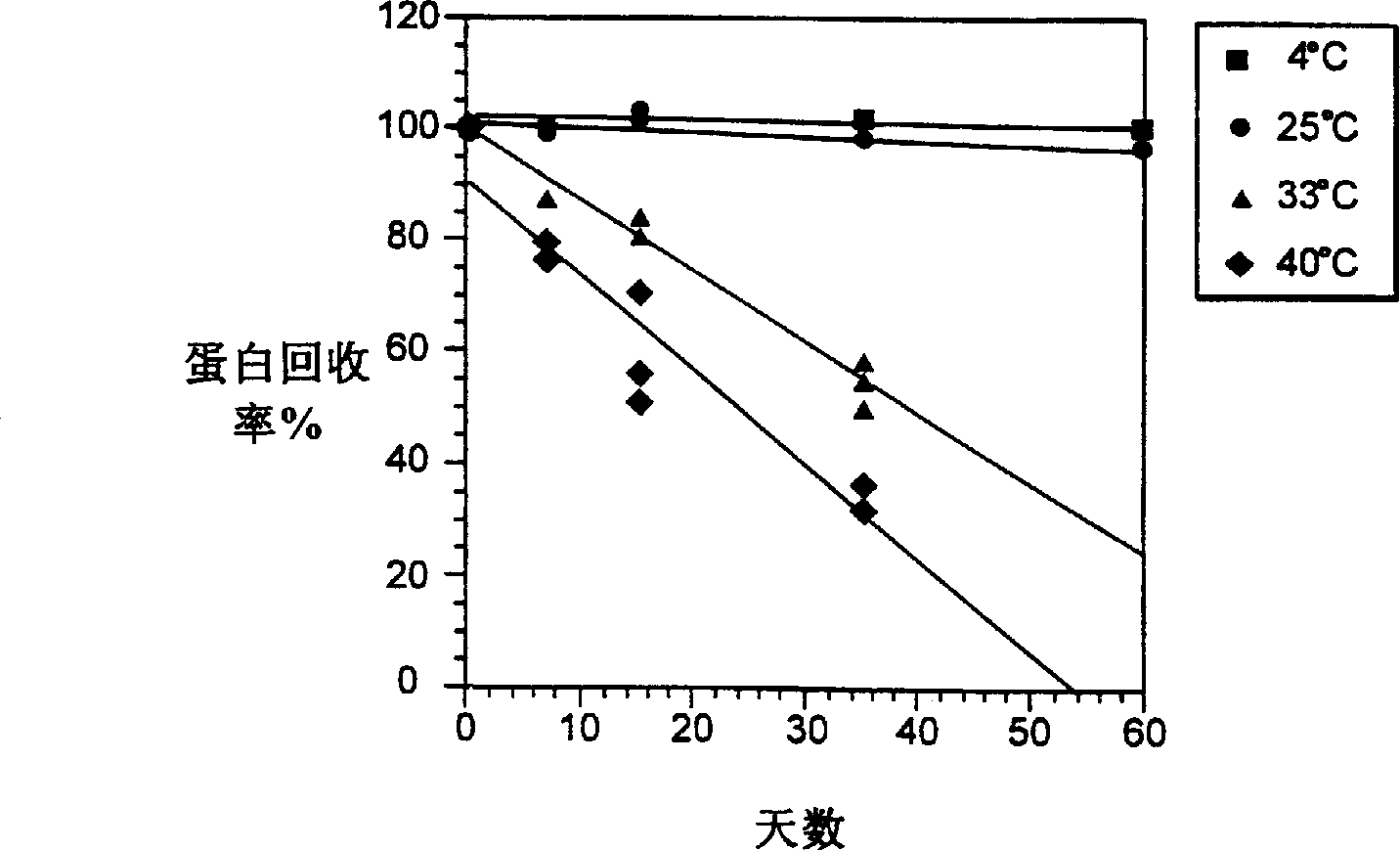
[0120] The presence / absence of insoluble aggregates was detected by measuring the absorbance at 320 nm and the transmittance at 580 nm. Soluble protein was determined by measuring the absorbance at 278-280 nm (extinction coefficient of 1.5), or by reversed-phase high-performance liquid chromatography (HPLC) using the known peak concentration of beta-interferon in the formulation buffer as a standard. concentration. The liquid formulation samples were centrifuged before the test. The percentage of soluble aggregates was determined by size exclusion chromatography on a TSK-Gel(R) G2000SWXL column (Toso Haas, Montgomeryville, PA) to separate aggregates from [beta]-interferon monomers. The peak area detected at 280 nm was used to ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com