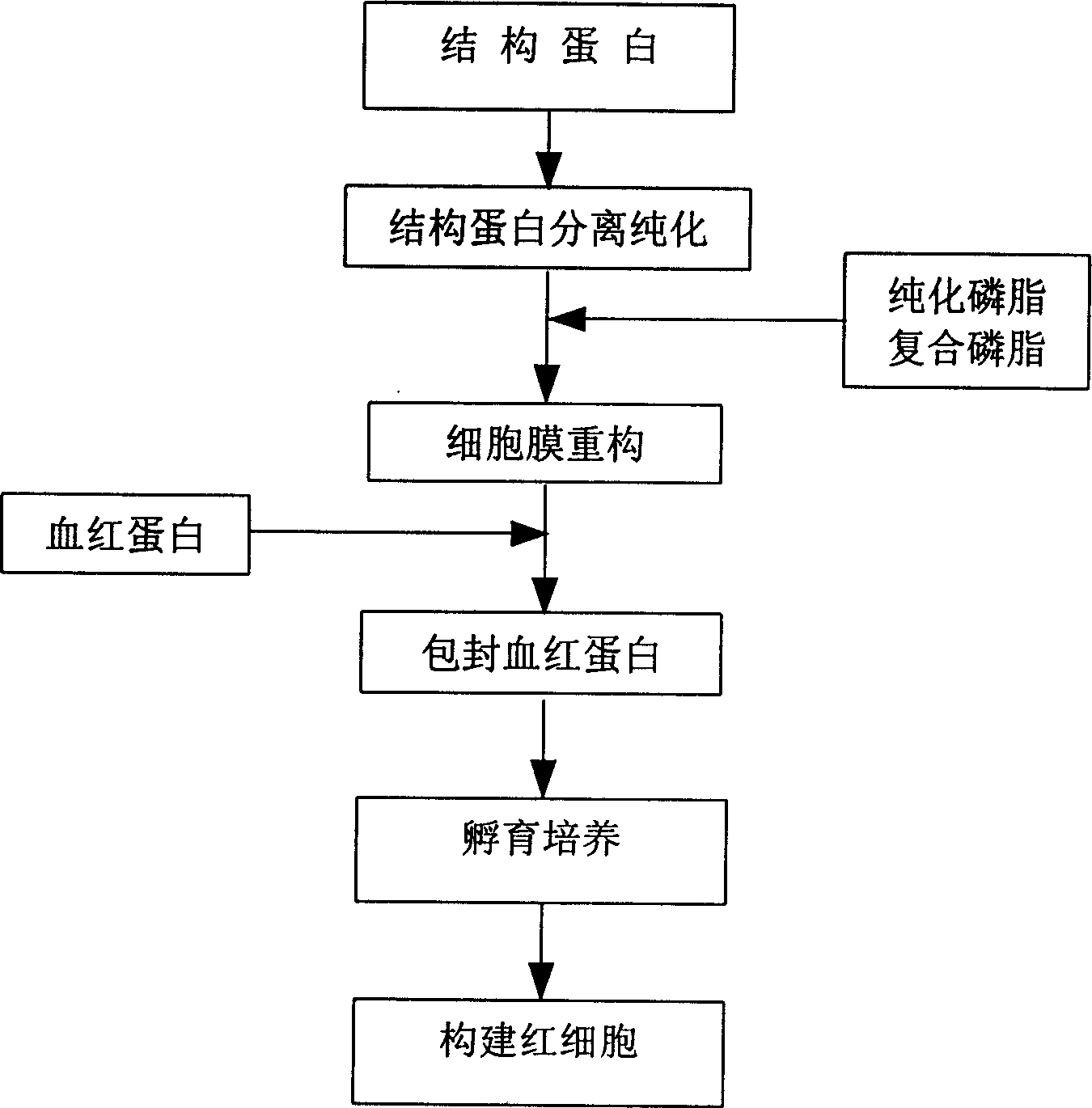
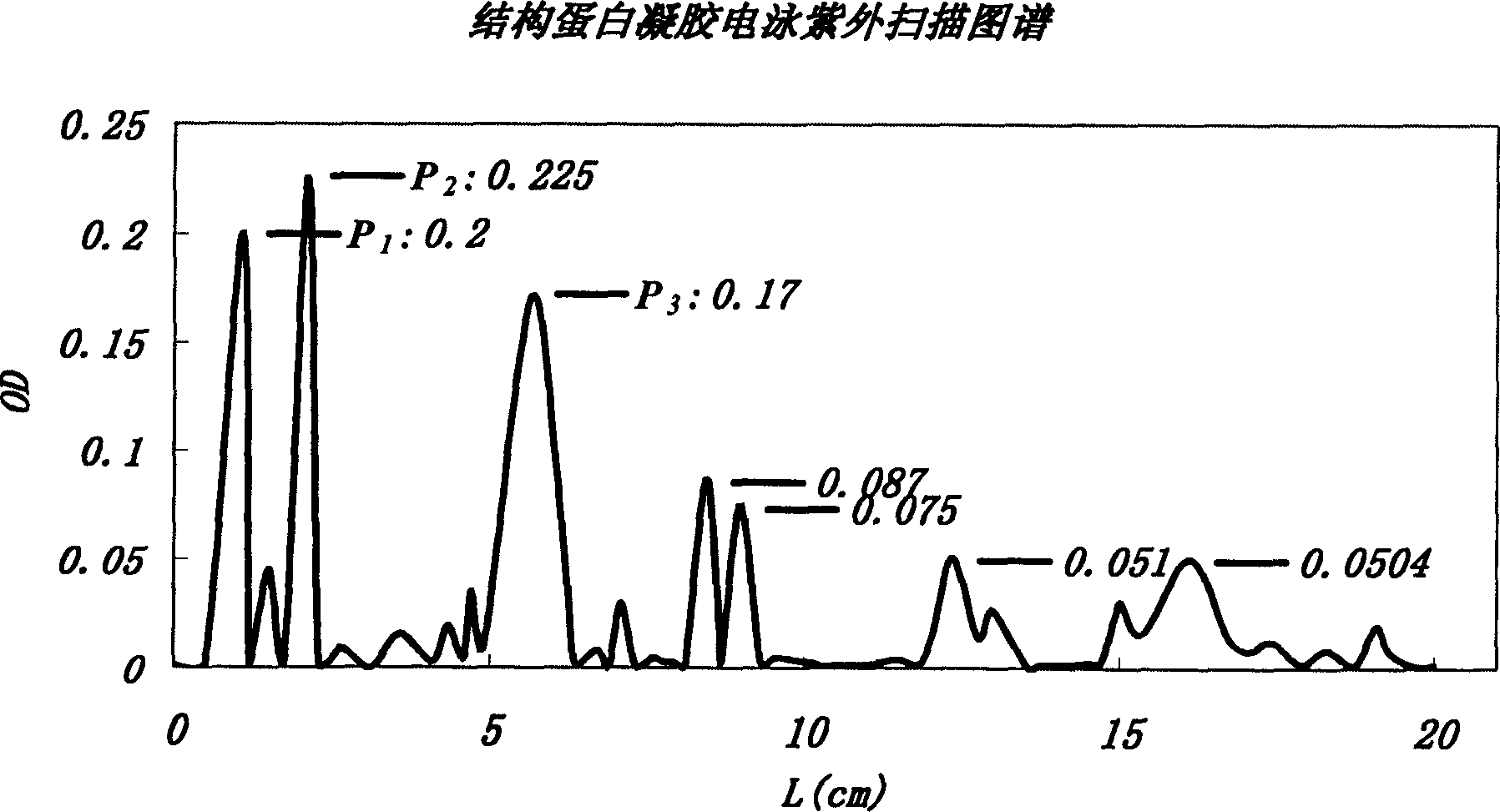
In vitro reconstituted human erythrocyte, its preparation and application in blood substitute material
A technology of human erythrocytes and erythrocytes, applied in animal cells, vertebrate cells, blood/immune system cells, etc., can solve the problems of brittle membranes, inestimable demand markets, difficulties in storage and transportation of natural blood, and improve life expectancy Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 Separation and Purification Method I of Structural Protein
[0034] (1), extract structural protein
[0035] Take 5 grams of soybeans and soak them in 10-30ml of physiological saline for 8-12 hours, mash the tissue and filter to remove the residue; add 10-200 120mmol magnesium chloride saline solution to the emulsion, and freeze and centrifuge the solution (5000rpm when flocs are formed) , ~4°C) for 15-20 minutes, discard the supernatant, dissolve the precipitate with normal saline, then add ammonium sulfate solution to saturate the coagulated flocs, and then centrifuge (5000rpm, ~4°C) for 15-20 minutes , to obtain crude 11S globulin.
[0036] (2) Separation and purification of structural proteins
[0037] The above crude 11S globulin was desalted by dialysis (molecular weight cut-off 2 + : 10~200mmol) to make more than 70% of the product be αβ dimer globulin.
Embodiment 2
[0038] Example 2 Separation and Purification of Structural Protein II
[0039] (1), extract structural protein
[0040] Use blood containing anticoagulants (approximately 5ml of heparin pH7.4 isotonic phosphate buffer per 30ml of blood) in order to minimize the denaturation and enzymatic hydrolysis of structural proteins, the entire operation should be carried out at a low temperature of 0-4°C . Take 5ml of the above-mentioned anticoagulated blood, refrigerated and centrifuged (3000rpm, ~4°C, 15-30 minutes) to precipitate red blood cells, and separate the plasma supernatant and buffy coat.
[0041] The erythrocytes were washed 2 to 5 times with 3 times the volume of pre-cooled pH7.4 isotonic phosphate buffer, each time refrigerated and centrifuged (3000rpm, ~4°C, 15 minutes), and the supernatant and precipitated surface layer were removed.
[0042] Add pre-cooled 5-10 mmol / L, pH 7.4 hypotonic Tris (trishydroxymethylaminomethane)-HCl buffer solution at a volume ratio of 10-50...
Embodiment 3
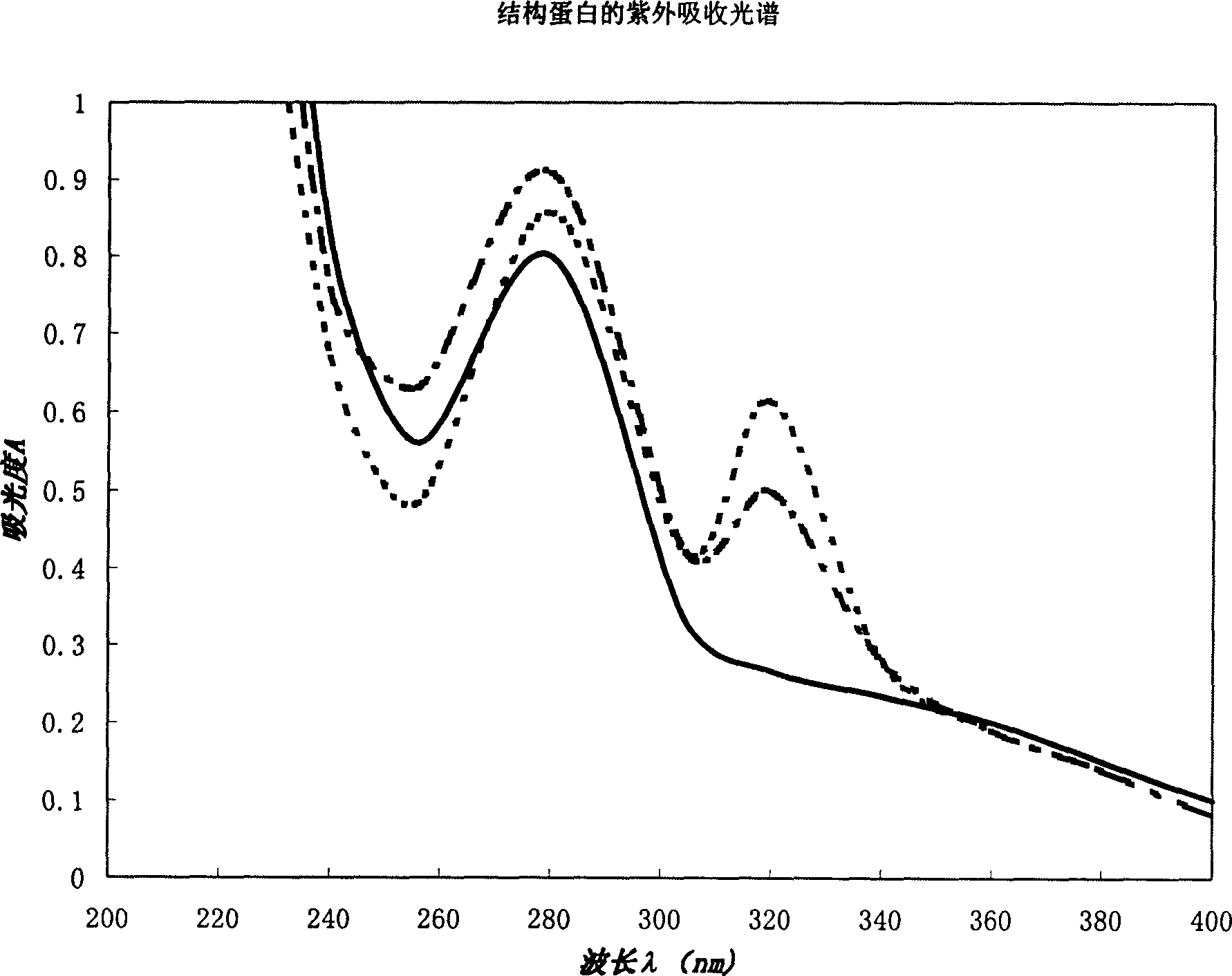
[0045] Embodiment 3 The ultraviolet absorption spectrum of structural protein
[0046] The structural protein product obtained by separation and purification is dissolved in physiological saline at a concentration of 200-800 ppm, and subjected to ultraviolet spectrophotometric analysis. Instrument: PE-λ900, scanning wavelength range: 200-400nm. For spectrogram see figure 2 . The solid lines in the figure represent the ultraviolet absorption peaks of membrane proteins derived from animal red blood cells, and the dotted lines represent the ultraviolet absorption peaks of 11S globulin derived from plant proteins. The three spectral curves all show the maximum absorption peak at 280nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com