Aspergillus niger strain and its culture method and application
A cultivation method, the technology of Aspergillus niger, which is applied in the direction of fungi, hydrolytic enzymes, fermentation, etc., can solve many problems, and achieve the effect of fast growth, not easy to mutate, and simple cultivation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] The cultivation of embodiment 1 thalline (1)
[0017] Use an inoculation loop to pick Aspergillus niger CGMCC 0496 thallus and connect it to the above-mentioned slant, grow in medium at 10-30°C for 2-3 days, and a large number of black spore groups appear. Then pick the well-grown bacteria from the slope and put them into the culture medium, culture them on a reciprocating shaker at 10-30°C for 2-3 days, centrifuge the obtained bacteria (5,000rmp) for 30 minutes, and wash the bacteria with 0.8% normal saline Wash, then centrifuge or filter to obtain the bacterial cells, and the obtained bacterial cells can be directly used for the reaction. The bacteria are gray spherical.
[0018] The slant medium contains 10-30g / L fructose, glucose or sucrose, 6-15g / L corn steep liquor, yeast extract or corn flour, 1-3g / L agar, 1.0g / L K 2 HPO 4 ·H 2 O, 0.5g / L KCl, 0.5g / L MgSO 4 ·H 2 O, 0.01g / L FeSO 4 ·H 2 O, adjust the pH value to 7 with inorganic bases such as ...
Embodiment 2
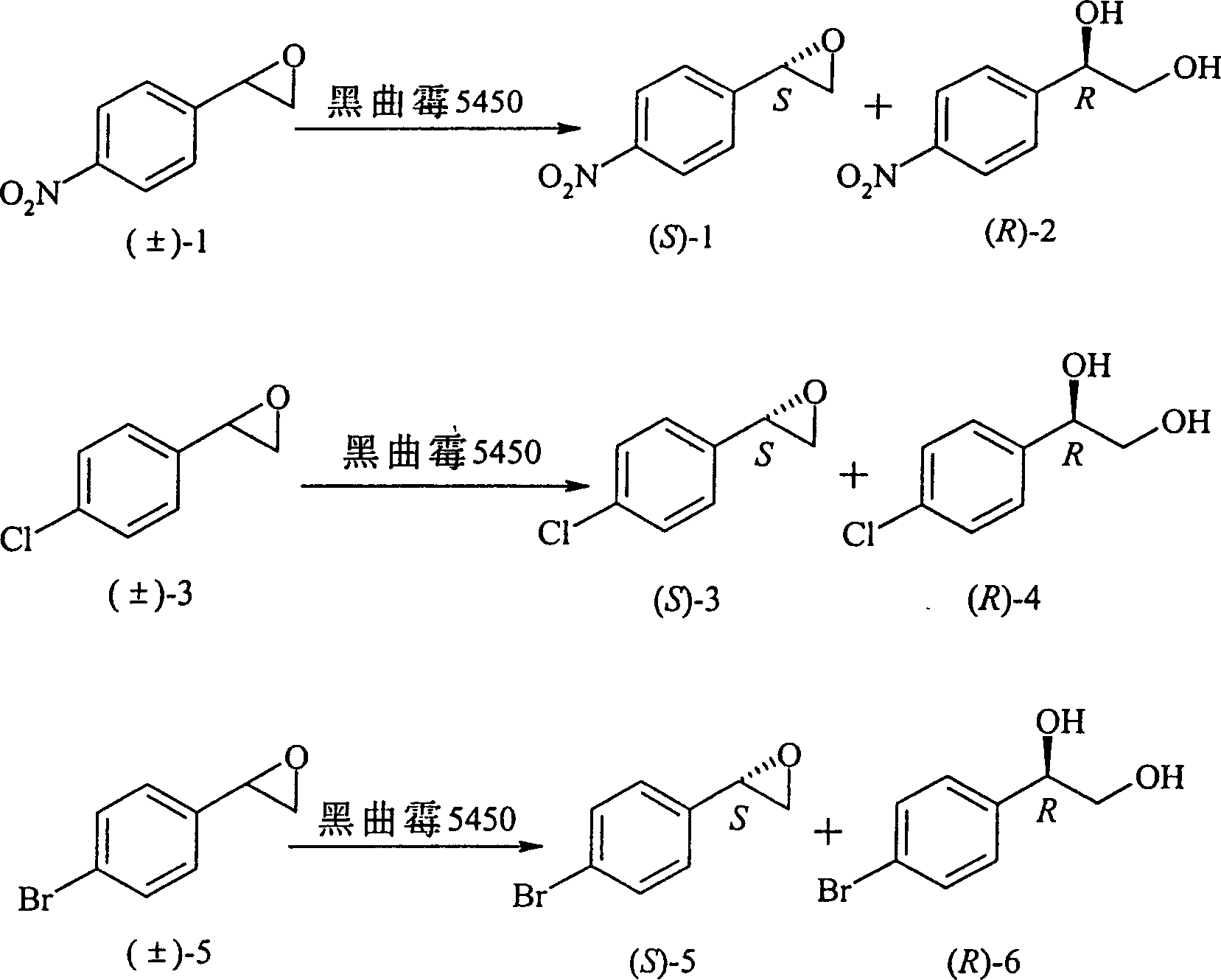
[0021] Example 2 Asymmetric Resolution of p-Chlorostyrene Oxirane
[0022] The product (S)-3 was obtained with a yield of 40% using p-chlorostyrene oxide as a substrate. Product (R)-4, yield 44%.
[0023] Product (S)-3: Optical purity: 96% [α] D 25 +19.2°(c1.0, CHCl 3 )
[0024] 1 NMR (CCl 4 +TMS, 60MHz) δ=2.6(dd, J 1 =6Hz,J 2 =2Hz, 1H), δ=3.1(dd, J 1 =6Hz,J 2 =4Hz, 1H), δ=3.6-3.8(m, 1H), δ=6.9-7.4(m, 4H)
[0025] MS m / z(rel.intensity%)156(M + +2, 2), 155 (M + +1, 3), 154 (M + , 8), 138(3), 125(40), 119(39), 91(29), 89(100)
[0026] Product (R)-4: Optical purity: 87% [G] D 25 -33.2° (c1.0, EtOH)
[0027] 1 NMR (CD 3 COCD 3+TMS, 300MHz) δ=3.13(bs, 2H), δ=3.53(dd, J 1 =10.9Hz,J 2 =7.4Hz, 1H), δ=3.61(dd, J 1 =11Hz,J 2 =4.6, 1H), δ=4.71(dd, J 1 =7.3Hz,J 2 =4.5Hz, 1H), δ=7.12-7.52(m, 4H)
[0028] MS m / z(rel.instensity%)172(M + , 2), 155(11), 141(86), 125(8), 113(26), 77(100), 51(21)
Embodiment 3
[0029] Example 3 Asymmetric resolution of p-bromostyrene oxide
[0030] The product (S)-5 was obtained with p-bromostyrene oxide as the substrate, and the yield was 35%. Product (R)-6, yield 43%.
[0031] Product (S)-5: Optical purity: 100% [α] D 25 +14.2° (c1.5, CHCl 3 )
[0032] 1 NMR (CCl 4 +TMS, 90MHz) δ=2.6(dd, J 1 =5Hz,J 2 =2Hz, 1H), δ=3.0(dd, J 1 =J 2 =4.5Hz, 1H), δ=3.6-3.7(m, 1H), δ=7.1-7.4(AB, J=6Hz, 4H)
[0033] MS m / z(rel.instensity%)200(M + +2, 3), 199 (M + +1, 3), 198 (M + , 4), 197(M+-1, 3), 169(14), 119(41), 89(100), 63(36)
[0034] Product (R)-6: Optical purity: 87% [α] D 25 -38.4° (c1.0, CHCl 3 )
[0035] 1 NMR (CD 3 COCD 3 +TMS, 300MHz) δ=3.58(bs, 2H), δ=3.88-3.94(m, 1H), δ=3.48(m, 1H), δ=4.71(m, 1H), δ=7.36-7.48(AB , J=8.4Hz, 4H)
[0036] MS m / z (rel.intensity %) 218, 216 (M + , 3), 187(71), 185(83), 159(16), 157(19), 77(100), 51(18)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com