Microbial limit detection method for azithromycin dry suspension
A microbial limit and dry suspension technology, applied in biochemical equipment and methods, microbial measurement/inspection, and resistance to vector-borne diseases, etc., can solve the impact of filtration speed, unspecified filtration speed, and detection accuracy of limit detection methods No high problems, to achieve the effect of increasing the dissolution time and strong elimination effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
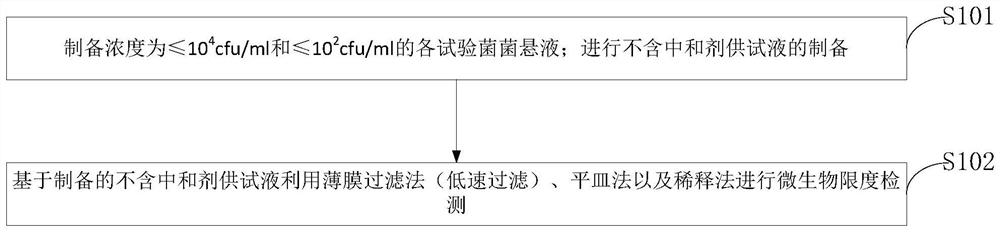
[0061] 1Materials and Instruments
[0062] 1.1 Test article
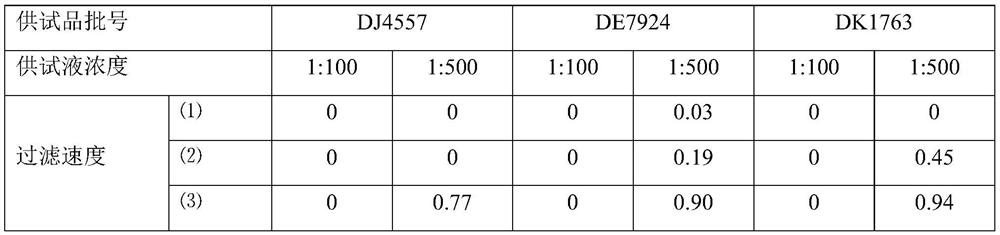
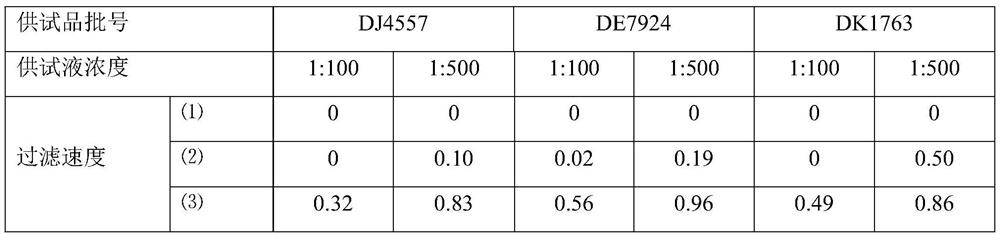
[0063] Azithromycin dry suspension, Pfizer Pharmaceutical Co., Ltd., specification: 0.1g, batch number: DJ4557, DE7924, DK1763.
[0064] 1.2 Instruments
[0065] SHP-160 biochemical incubator and GNP-9160E water-proof incubator are produced by Shanghai Sanfa Scientific Instrument Co., Ltd.; INE500 electric heating constant temperature incubator is produced by German MEMMERT company; HTY-302G microbial limit tester is produced by Hangzhou Tai Lin Biotechnology Equipment Co., Ltd.; TD electronic balances are produced by Yuyao Jinnuo Balance Instrument Co., Ltd.; RH basic 2 magnetic stirrers are produced by German IKA company.
[0066] 1.3 Bacteria
[0067] Staphylococcus aureus [CMCC(B) 26 003], Bacillus subtilis [CMCC(B) 63 501], Pseudomonas aeruginosa [CMCC(B) 10 104], Candida albicans [CMCC(F) 98 001] , Aspergillus niger [CMCC (F) 98 003], Escherichia coli [CMCC (B) 44 102]. The working strains used were the t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com