Disulfide bond compound as well as preparation method and antibacterial application thereof
A compound and disulfide bond technology, applied in the field of disulfide bond compounds and their preparation methods and antibacterial applications, can solve the problems of lack of chemical agents, enhanced microbial resistance, etc., and achieve good antifungal and bacterial effects active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: the synthesis of compound S1
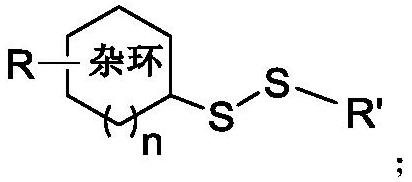
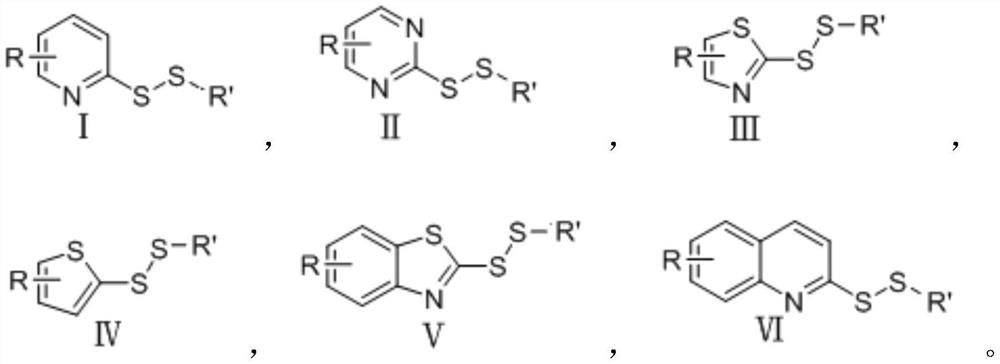
[0039] (1) The structural formula of compound S1 is as follows:
[0040]
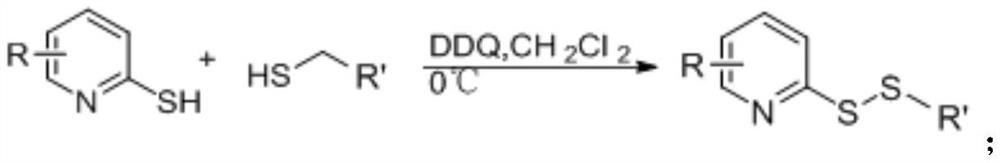
[0041] (2) the synthetic reaction formula of compound S1 is as follows:
[0042]
[0043] (3) the preparation method of compound S1 is as follows:
[0044] 2-Mercaptopyridine (1 mmol) and n-butanethiol (1.5 mmol) were dissolved in 10 mL of dichloromethane (DCM) at 0°C. 1 mmol of dichlorodicyanobenzoquinone (DDQ) was then slowly added to the solution in portions and the reaction mixture was stirred in an ice bath for 1 h. The progress of the reaction was monitored by thin layer chromatography. Upon completion, the solvent was removed by rotary evaporation and the crude compound was purified by column chromatography (ethyl acetate:petroleum ether, 1:50) to give the desired product S1.
[0045] (4) Results:
[0046] The obtained compound S1 is a colorless oily liquid, yield: 82%; 1 H NMR (400MHz, Chloroform-d) δ: 8.52-8.40 (m, 1H), 7.80-7.55 (...
Embodiment 2
[0047] Embodiment 2: the synthesis of compound S2
[0048] (1) The structural formula of compound S2 is as follows:
[0049]
[0050] (2) the preparation method of compound S2 is as follows:
[0051] The difference between the preparation method and Example 1 is that only n-propanethiol is used to replace the n-butanethiol of Example 1, and the others are the same as those of Example 1.
[0052] (3) Results:
[0053] The obtained compound S2 is a colorless oily liquid, yield: 85%; 1 H NMR (400MHz, Chloroform-d)δ: 8.47-8.42(m,1H), 7.75-7.70(m,1H), 7.66-7.60(m,1H), 7.08-7.03(m,1H), 2.76(t) , J=7.3Hz, 2H), 1.71(h, J=7.3Hz, 2H), 0.98(t, J=7.4Hz, 3H). 13 C NMR (100MHz, Chloroform-d) δ: 160.85, 149.65, 137.03, 120.55, 119.65, 41.09, 22.42, 13.21. MS-ESI m / z: C 8 H 11 NS 2 [M+H] + : 186.3030.
Embodiment 3
[0054] Embodiment 3: the synthesis of compound S3
[0055] (1) the structural formula of compound S3 is as follows:
[0056]
[0057] (2) the preparation method of compound S3 is as follows:
[0058] The difference between the preparation method and Example 1 is that only 2-mercaptopyrimidine is used to replace the 2-mercaptopyridine of Example 1, and n-propanethiol is used to replace the n-butanethiol of Example 1. Others are the same as those of Example 1.
[0059] (3) Results:
[0060] The obtained compound S3 is a colorless oily liquid, yield: 77%; 1 H NMR (400MHz, Chloroform-d) δ: 8.60 (d, J=4.8Hz, 2H), 7.07 (t, J=4.8Hz, 1H), 2.83 (t, J=7.3Hz, 2H), 1.73 (h) , J=7.3Hz, 2H), 0.99(t, J=7.3Hz, 3H). 13 C NMR (100MHz, Chloroform-d) δ: 172.16, 157.92, 117.89, 40.68, 22.21, 13.22. MS-ESI m / z: C 7 H 10 N 2 S 2 [M+H] + : 187.2910.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com