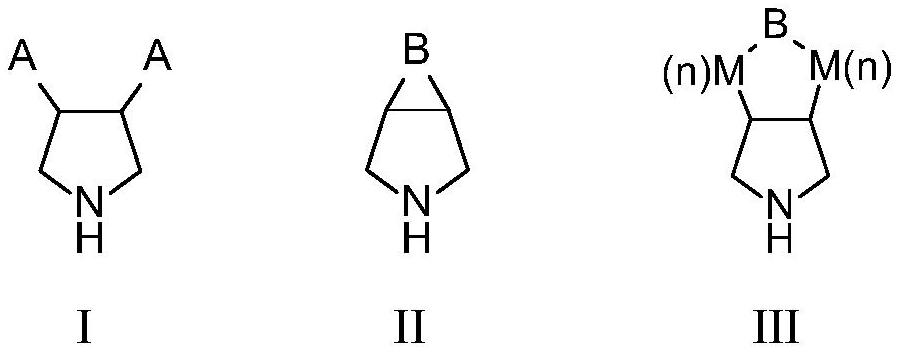
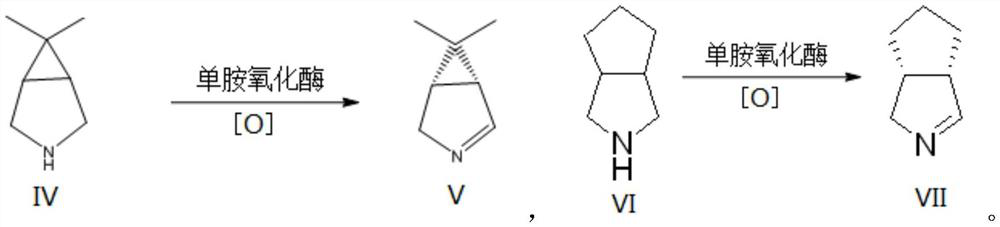
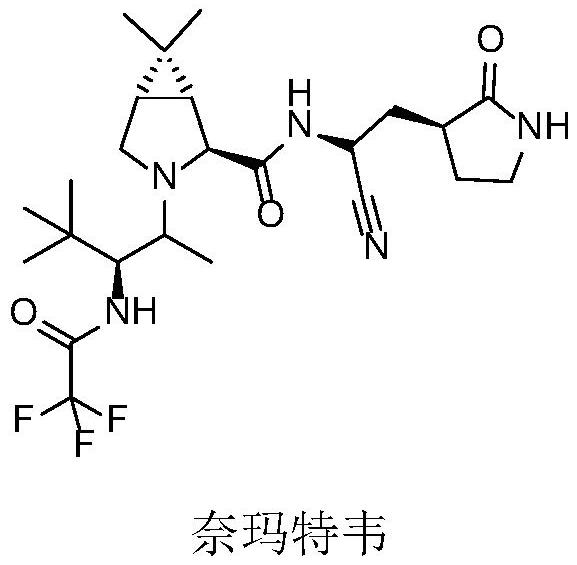
Monoamine oxidase and application thereof
A monoamine oxidase, oxidation reaction technology, applied in applications, enzymes, biochemical equipment and methods, etc., can solve the problems of low substrate concentration, low activity of MAON401, and difficulty in realizing industrial-scale application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Example 1: Gene cloning of monoamine oxidase
[0086] The monoamine oxidase with obvious activity to 6,6-dimethyl-3-azabicyclo[3.1.0]hexane was predicted by bioinformatics analysis method, and then cloned and expressed to verify its function. Using this method, three novel monoamine oxidase genes were cloned from Penicillium sp., Zymoseptoria sp. and Alternaria sp., respectively.
[0087] As an example, using the genomic DNA of Penicillium as a template, a complete nucleic acid molecule encoding the monoamine oxidase SEQ ID No. 1 is obtained by using conventional technical methods in the art (such as polymerase chain reaction PCR). The synthetic primers involved are:
[0088] Upstream primer: CCG GAATTC atgacctctcgcgacggt,
[0089] Downstream primer: CCG CTCGAG tagccgaagccgtgg.
[0090]The underlined sequence of the upstream primer is the restriction site of EcoR I, and the underlined sequence of the downstream primer is the restriction site of Xho I.
[0091] U...
Embodiment 2
[0094] Example 2: Preparation of monoamine oxidase recombinant expression vector and recombinant expression transformants
[0095] The monoamine oxidase gene obtained by PCR amplification in Example 1 and the empty vector plasmid pET28a were double digested with restriction enzymes EcoR I and Xho I at 37°C for 12h respectively. The double-enzyme digested product was verified by agarose gel electrophoresis, and then purified and recovered. The obtained linearized pET28a plasmid was ligated with the purified target gene fragment with T4 DNA ligase at 16 °C overnight, and the ligated product was transformed into the large intestine. Bacillus E.coli BL21 (DE3) competent cells (Novagen), and evenly spread on LB agar plates containing 50 μg / mL kanamycin, placed in a 37 ° C incubator for about 12 hours, and the long The colonies that came out were verified by colony PCR, and clones with positive colony PCR were picked for sequencing verification. After the sequence was verified to b...
Embodiment 3
[0099] Example 3: Construction of monoamine oxidase mutants and high-throughput plate screening
[0100] Monoamine oxidase PsMAO was used as the parent, and error-prone PCR technology was used to construct a random mutant library of monoamine oxidase: using pET28a-PsMAO as a template and the upstream primer and downstream primer in Example 1 as primer pairs, error-prone PCR was performed with rTaq DNA polymerase , to build a random mutation library. PCR system (50 μL): 0.5 μL of rTaq DNA polymerase, 10×PCR buffer (Mg 2+ Plus) 5.0 μL, dNTP Mixture (2.0 mM each) 4.0 μL, MnCl with a final concentration of 100 μmol / L 2 , pET28a-PsMAO plasmid 0.5ng, upstream and downstream primers (10μM) each 2μL, add sterilized distilled water to make up to 50μL. PCR reaction procedure: (1) pre-denaturation at 95°C for 5 min; (2) denaturation at 94°C for 30s; (3) annealing at 55°C for 30s; (4) extension at 72°C for 2 min; steps (2) to (4) were carried out for a total of 30 cycles ; The final ex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com