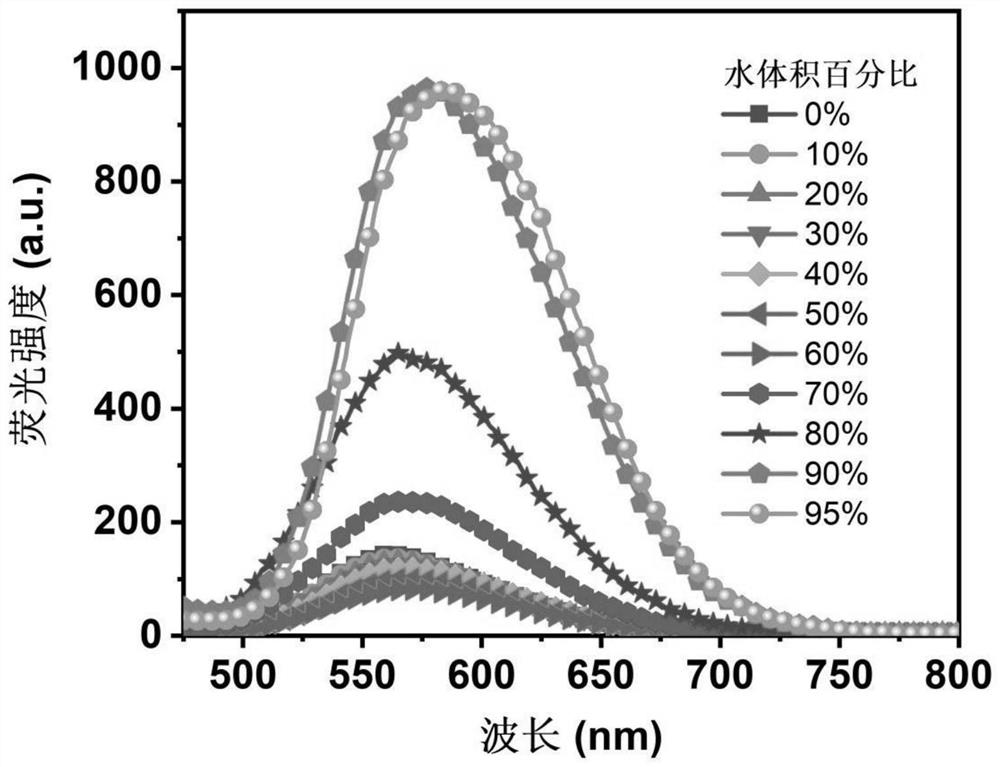
Fluorescent compound for detecting automobile brake fluid as well as preparation method and application of fluorescent compound
A technology for fluorescent compounds and automobile braking, applied in the field of fluorescent probe detection, can solve problems such as detection application limitations, and achieve the effects of good visibility, mild conditions, and simple and reliable detection methods.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Combine 4-(di-p-tolylamino)benzaldehyde (6.02 g, 20 mmol) with (E)-4-(((4'-(cyanomethyl)-[1,1'-diphenyl]-4-yl )methylene)amino)benzonitrile (7.16 g, 26 mmol) was dissolved in 100 mL of acetonitrile, and 3 drops of piperidine were added dropwise. After the feeding was completed, the reaction mixture was stirred at 80°C for 6 hours. After the heating was stopped, the reaction solution was filtered under reduced pressure, and the filter cake was washed with acetonitrile at 0-10°C. The filter cake was collected and recrystallized from acetonitrile. The recrystallized product was filtered, the obtained solid was collected and dried in vacuo to obtain a total of 9.78 g of the product DBPT-3 with a yield of 81%.
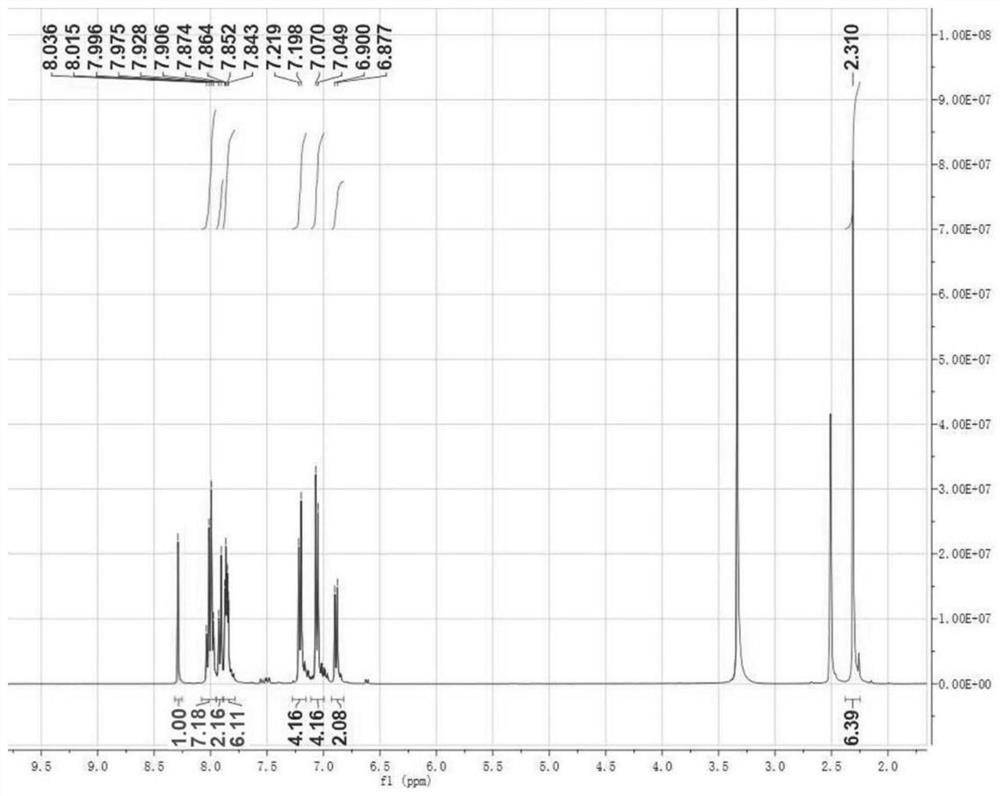
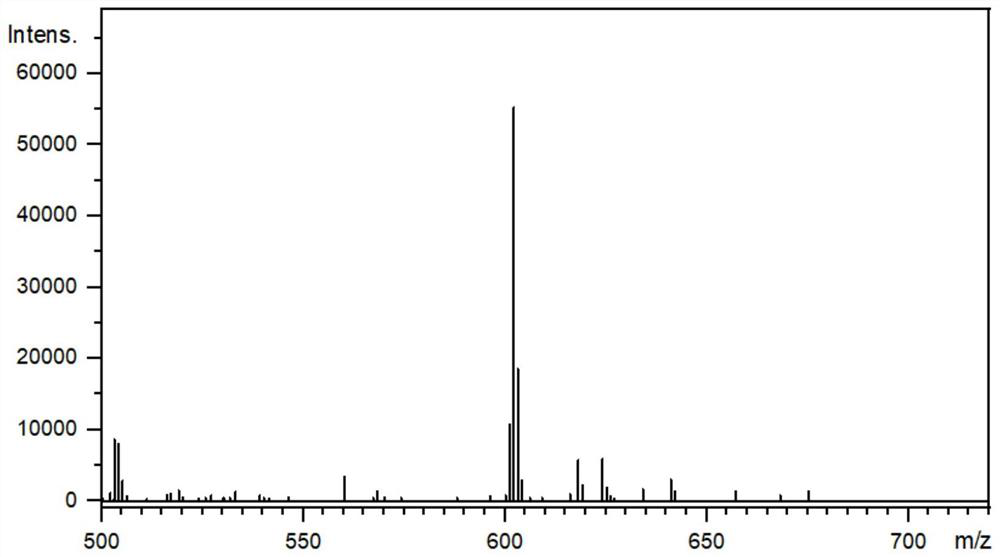
[0051] The structural characterization data related to compound DBPT-3 are as follows: 1 H NMR (400MHz, DMSO-d 6 ,δ):8.29(s,1H),8.05-7.98(m,7H),7.91(d,J=8.8,2H),7.87-7.84(m,6H),7.21(d,J=8.4,4H) , 7.06(d, J=8.4, 4H), 6.89(d, J=9.2, 2H), 2.31(s, 6H). H NMR spectru...
Embodiment 2
[0053] Combine (Z)-2-(4-bromophenyl)-3-(4-(di-p-tolylamino)phenyl)acrylonitrile (4.78 g, 10 mmol) with (E)-(4-((((4- Cyanophenyl)imino)methyl)phenyl)boronic acid (2.76g, 11mmol) was dissolved in 100mL of tetrahydrofuran / water mixed (volume ratio 4:1) solution, and 0.067g of palladium acetate was added. After the feeding was completed, the reaction system was deoxygenated, and then the reaction mixture was stirred at 100° C. for 12 hours under a nitrogen atmosphere. After the heating was stopped, the reaction solution was cooled to room temperature and the insolubles were filtered, then an equal volume of water was added and the aqueous phase was extracted with dichloromethane. Combine the organic phases, spin dry, and then perform silica gel column chromatography with dichloromethane:petroleum ether:ethyl acetate=1:6:0.5 to 1:4:1 (volume ratio) as the fluidity, after gradient elution A total of 4.53 g of the product DBPT-3 was obtained with a yield of 75%. The structural cha...
Embodiment 3
[0055] (Z)-3-(4-(Di-p-tolylamino)phenyl)-2-(4'-formyl-[1,1'-diphenyl]-4-yl)acrylonitrile (0.50 g, 1 mmol) and 4-aminobenzonitrile (0.18 g, 1.5 mmol) were dissolved in 50 mL of ethanol, and 3 drops of glacial acetic acid were added dropwise. After the feeding was completed, the reaction mixture was stirred at room temperature for 10 hours. After the reaction is heated, the reaction solution is filtered under reduced pressure, and the filter cake is washed with ethanol at 0-10° C. (the solvent is less volatile at low temperature, and the solubility of the reactant and the product is quite different). The filter cake was collected and recrystallized from ethanol. The recrystallized product was filtered, and the obtained solid was collected and dried in vacuo to obtain a total of 0.54 g of the product DBPT-3, with a yield of 90%. The structural characterization results of the compound DBPT-3 synthesized by this method are the same as those in method 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com