A kind of benzoxazole solid fluorescent material
A technology of benzoxazoles and fluorescent materials, applied in the field of fluorescent materials, can solve the problems of low efficiency, lack of blue-light material building groups, low color purity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Synthesis of compound BZ-1
[0038] Structural formula:
[0039]
[0040] Chinese name: 2-phenoxybenzo[d]oxazole
[0041] English name: 2-phenoxybenzo[d]oxazole
[0042] Synthesis method: dissolve phenol (0.6110g, 6.48mmol) in 20ml of acetonitrile, add anhydrous potassium carbonate (0.8960g, 6.48mmol), and reflux for 1h. 2-Chlorobenzoxazole (0.9060 g, 5.9 mmol) was added to the above solution, and the heating was continued for 4 h under reflux. After the reaction was completed, suction filtration was performed to obtain the filtrate, and the organic solvent was spun off to obtain a crude product. Add n-hexane for recrystallization to obtain 0.87 g of a white solid product with a yield of 70%. 1 H NMR (500MHz, CDCl 3 )δ7.52(d, J=7.4Hz, 1H), 7.50–7.45 (m, 2H), 7.45–7.40 (m, 3H), 7.31 (d, J=7.3Hz, 1H), 7.29–7.21 (m , 2H). 13 C NMR (126MHz, CDCl 3 )δ161.20,151.73,147.39,139.72,128.93,125.40,123.48,122.39,119.12,117.72,108.85.
Embodiment 2
[0043] Example 2: Synthesis of Compound BZ-2
[0044]
[0045] Chinese name: 2-(4-methoxyphenoxy)benzo[d]oxazole
[0046] English name: 2-(4-methoxyphenoxy)benzo[d]oxazole
[0047] Synthesis method: Dissolve 4-methoxyphenol (0.4450g, 3.58mmol) in 20ml of acetonitrile, add anhydrous potassium carbonate (0.4950g, 3.58mmol), and reflux for 1h. 2-Chlorobenzoxazole (0.5 g, 3.26 mmol) was added to the above solution, and the heating was continued for 4 h under reflux. After the reaction was completed, dichloromethane was added for suction filtration to obtain a filtrate, and the organic solvent was spun off to obtain a crude product. Add n-hexane for recrystallization to obtain 0.5260 g of a white solid product with a yield of 67%. 1 H NMR (500MHz, CDCl 3 )δ7.50(d,J=7.4Hz,1H),7.41(d,J=9.1Hz,1H),7.32(d,J=9.0Hz,2H),7.28–7.20(m,2H),6.97( d, J=9.0Hz, 2H). 13 C NMR (126MHz, CDCl 3 )δ162.82,157.65,148.44,146.28,140.77,124.41,123.24,121.22,118.64,114.85,109.80,55.63.
Embodiment 3
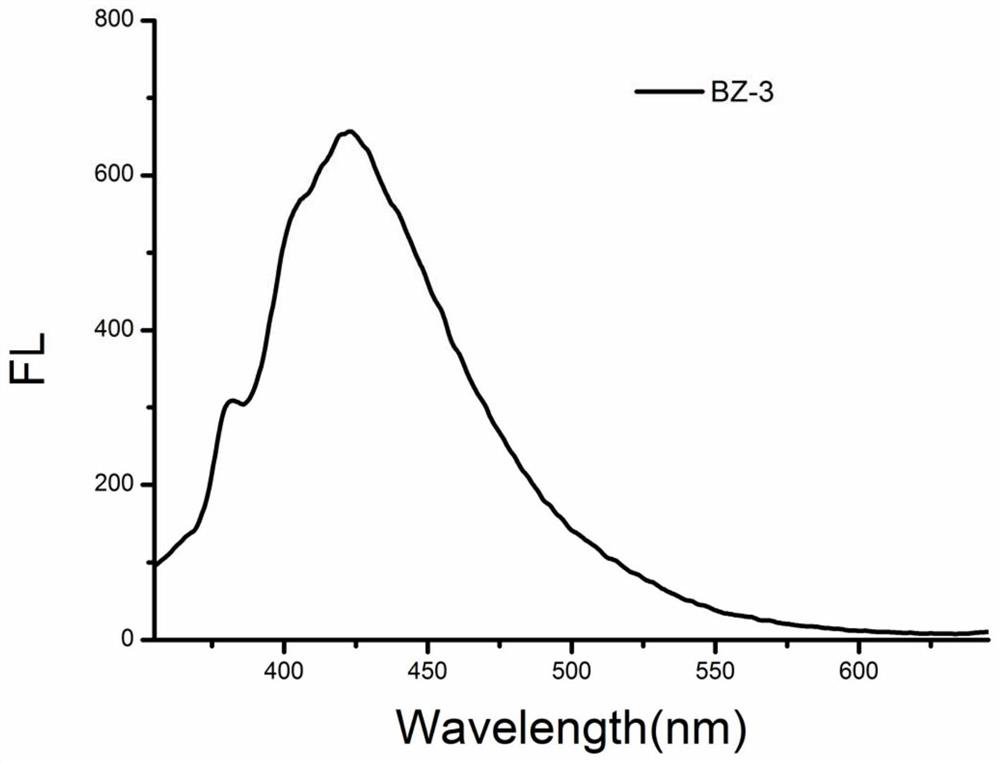
[0048] Example 3: Synthesis of compound BZ-3
[0049]
[0050]Chinese name: 2-(4-Chlorophenoxy)benzo[d]oxazole
[0051] English name: 2-(4-chlorophenoxy)benzo[d]oxazole
[0052] Synthesis method: Dissolve 4-chlorophenol (0.4610g, 3.59mmol) in 20ml of acetonitrile, add anhydrous potassium carbonate (0.4950g, 3.58mmol), and reflux for 1h. 2-Chlorobenzoxazole (0.5 g, 3.26 mmol) was added to the above solution, and the heating was continued for 4 h under reflux. After the reaction was completed, dichloromethane was added for suction filtration to obtain a filtrate, and the organic solvent was spun off to obtain a crude product. Add n-hexane for recrystallization to obtain 0.52 g of a white solid product with a yield of 65%. 1 H NMR (500MHz, CDCl 3 )δ7.52(d,J=8.9Hz,1H),7.45-7.41(m,3H),7.39(d,J=9.0Hz,2H),7.30-7.23(m,2H). 13 C NMR (126MHz, CDCl 3 )δ160.85,150.10,147.38,139.49,130.82,128.97,123.62,122.61,120.55,117.76,108.93.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com