Method for producing silver microparticles
A manufacturing method and technology of silver particles, applied in transportation and packaging, photovoltaic power generation, metal processing equipment, etc., can solve the problems of silver particle shape and property change, seriousness, precipitation, etc., and achieve sufficient continuous production and quality uniformity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
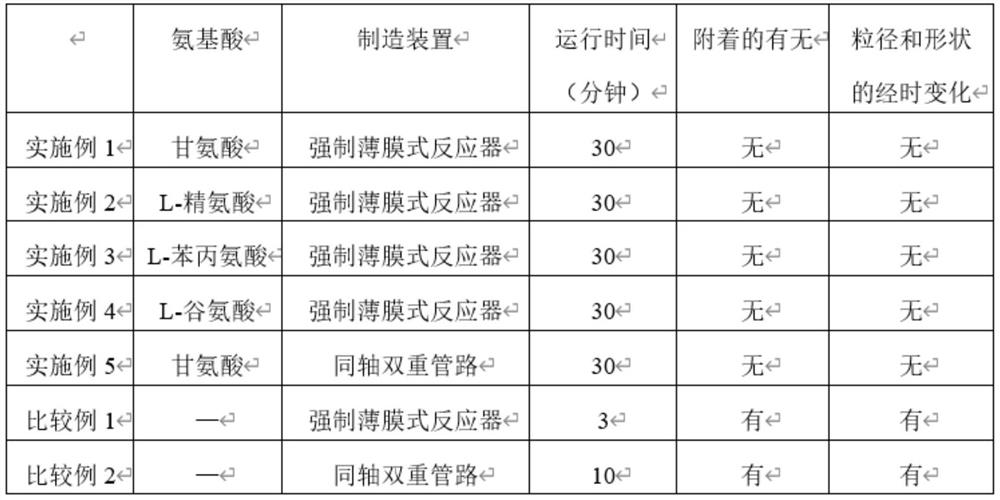
Embodiment 1
[0074] By adding 250 parts of silver nitrate (manufactured by Kishida Chemical Co., Ltd.) as a silver compound, 111 parts of glycine (manufactured by Kanto Chemical Co., Ltd.) as an amino acid (with respect to 1 mol of silver) to 9589 parts of ion-exchanged water ion is 1 mol) and 50 parts of 60% nitric acid (manufactured by Kishida Chemical Co., Ltd.) as a pH adjuster, and dissolved in an air atmosphere at 25°C to obtain a silver compound aqueous solution (1).
[0075] By adding 150 parts of hydrazine monohydrate (manufactured by Kanto Chemical Co., Ltd.) as a reducing agent and 5 parts of a 10% methanol solution of oleic acid as a dispersant to 845 parts of ion-exchanged water, the dispersant was prepared by adding oleic acid ( (manufactured by Kanto Chemical Co., Ltd.) was dissolved in methanol, and dissolved in an air atmosphere of 25° C. to obtain an aqueous reducing agent solution (1).
[0076] Using a forced thin film reactor ULREA (manufactured by M Technique Co., Ltd....
Embodiment 2
[0080] By adding 250 parts of silver nitrate (manufactured by Kishida Chemical Co., Ltd.) as a silver compound, 52 parts of L-arginine (manufactured by Fujifilm Wako Pure Chemical Industries, Ltd.) as an amino acid to 9693 parts of ion-exchanged water ) (0.2 mol per 1 mol of silver ions) and 5 parts of 60% nitric acid (manufactured by Kishida Chemical Co., Ltd.) as a pH adjuster, and dissolved in an air atmosphere at 25° C. to obtain a silver compound Aqueous solution (2).
[0081] By adding 100 parts of hydrazine monohydrate (manufactured by Kanto Chemical Co., Ltd.) as a reducing agent and 5 parts of a 10% methanol solution of oleic acid as a dispersant to 895 parts of ion-exchanged water, the dispersant was prepared by adding oleic acid ( (manufactured by Kanto Chemical Co., Ltd.) was prepared by dissolving in methanol, and dissolved in an air atmosphere of 25° C. to obtain an aqueous reducing agent solution (2).
[0082] Using a forced thin film reactor ULREA (manufacture...
Embodiment 3
[0086] By adding 250 parts of silver nitrate (manufactured by Kishida Chemical Co., Ltd.) as a silver compound, 243 parts of L-phenylalanine (manufactured by Fujifilm Wako Pure Chemical Industries, Ltd.) as an amino acid to 9502 parts of ion-exchanged water manufacture) (1 mol relative to 1 mol of silver ions) and 5 parts of 60% nitric acid (manufactured by Kishida Chemical Co., Ltd.) as a pH adjuster, and dissolved in an air atmosphere at 25° C. to obtain a silver compound Aqueous solution (3).
[0087] By adding 225 parts of hydrazine monohydrate (manufactured by Kanto Chemical Co., Ltd.) as a reducing agent and 5 parts of a 10% methanol solution of oleic acid as a dispersant to 770 parts of ion-exchanged water, the dispersant was prepared by adding oleic acid ( (manufactured by Kanto Chemical Co., Ltd.) was prepared by dissolving in methanol, and dissolved in an air atmosphere of 25° C. to obtain an aqueous reducing agent solution (3).
[0088] Using a forced thin film rea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com