Method for synthesizing nitroalkane at low temperature
A technology for nitroalkane and nitrohexane, which is applied in the field of chemical engineering, can solve the problems of high reaction temperature, high pressure, alkane fracture, etc., and achieves the effects of cheap and easy-to-obtain reaction raw materials and low reaction temperature.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
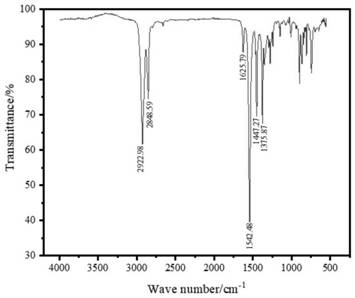
Image
Examples
Embodiment 1
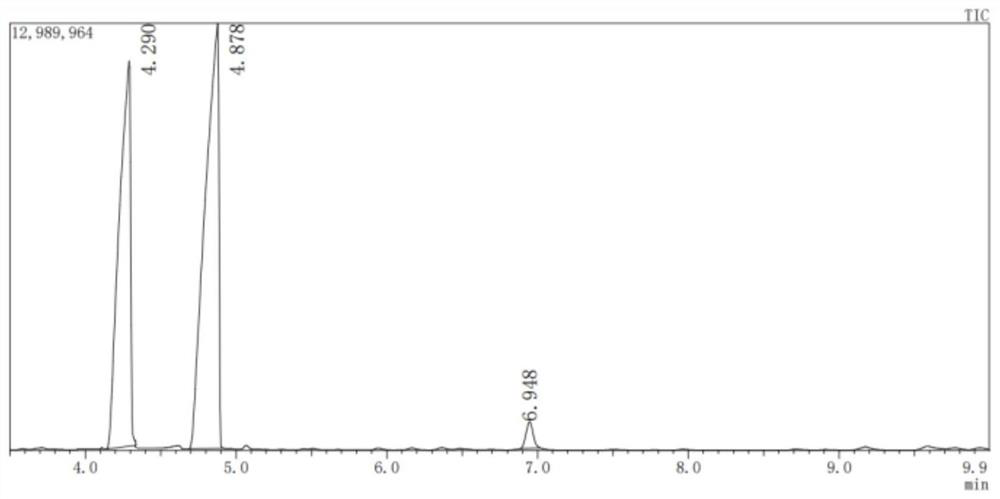
[0037] Accurately pipette 4.31g of n-hexane, 1.293g of TBP and 8.33mL of 2mol L into the Teflon liner -1 HNO 3 , then put the magnet into the PTFE liner and transfer the liner to the kettle sleeve for fixing and sealing, and finally put the kettle sleeve into the heating base, connect the thermocouple and pressure sensor, and set the reaction The temperature is 100°C, and the reaction time is 6h. After the reaction, the sample was taken out, and the sample was washed with alkali with sodium bicarbonate solution, repeated three times, and finally the sample was dried with anhydrous sodium sulfate. The product was then analyzed by GC-MS. The yields of the three nitro compounds were 5.9% for 1-nitrohexane, 33.1% for 2-nitrohexane and 26.3% for 3-nitrohexane.
Embodiment 2
[0039] Process and used reactor are the same as embodiment 1, and difference is that the substance concentration of nitric acid is 10mol / L, and the productive rate of three kinds of nitro compounds is respectively 1-nitrohexane 10.3%, 2-nitrohexane 39.4%, 3-nitrohexane 33.2%.
Embodiment 3
[0041] The process and the reactor used are the same as in Example 1, except that the reaction temperature is 130°C, and the yields of the three nitro compounds are respectively 9.9% for 1-nitrohexane, 37.9% for 2-nitrohexane, and 37.9% for 3-nitrohexane. Nitrohexane 30.6%.
[0042] By embodiment 1 and embodiment 2, illustrate that the nitric acid concentration is higher, and the productive rate of nitrohexane is higher.
[0043] Through embodiment 1 and embodiment 3, illustrate that reaction temperature is higher, and the productive rate of nitrohexane is higher.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com