Recombinant escherichia coli capable of producing L-methionine at high yield without action of exogenous amino acid and application of recombinant escherichia coli
A technology for recombining Escherichia coli and exogenous amino acids, applied in application, recombinant DNA technology, microorganism-based methods, etc., to achieve the effect of enhancing utilization capacity, improving energy and precursor substances, and reducing operational difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
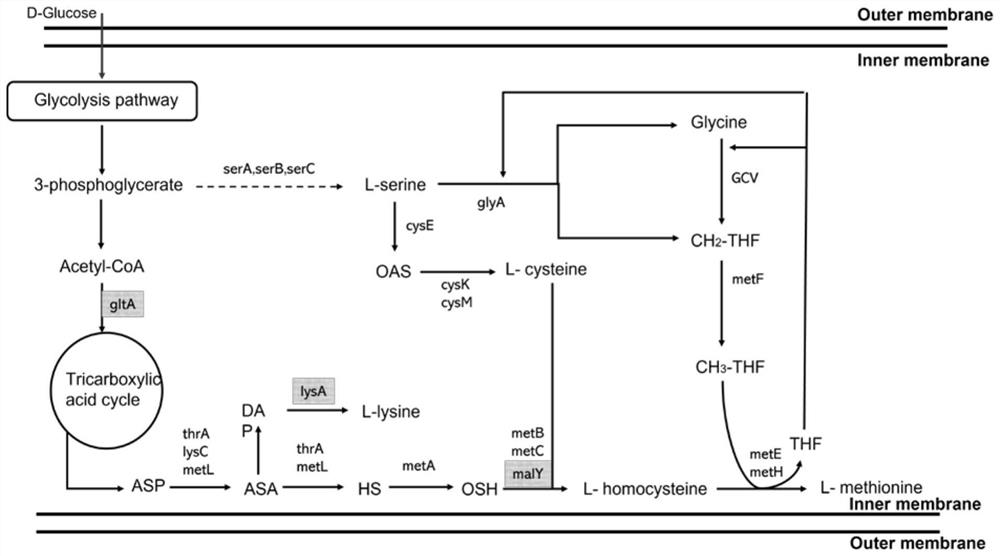
Image
Examples
Embodiment 1
[0042] Example 1: Fermentation method and content determination of L-methionine high-yielding strain
[0043] 1. Fermentation method:
[0044] Inoculate the constructed strain into 10 mL of LB medium containing 50 mg / L kanamycin (Kan), culture at 37°C for 8-12 hours, and then inoculate the culture solution to 20 mL containing 50 mg / L In the Kan's fermentation medium, the fermentation culture was carried out at 30° C. and 180 rpm for 48 hours to obtain a fermentation broth.
[0045] The composition of the fermentation medium is as follows: glucose 20g / L, (NH 4 ) 2 SO 4 16g / L, KH 2 PO 4 1g / L, Na 2 S 2 o 3 2g / L, yeast extract 2g / L, CaCO 3 10g / L, VB 12 0.2μg / L, 1mL / L trace element solution, the solvent is deionized water, the pH value is natural, and CaCO 3 and VB 12 Added at the time of inoculation; the composition of the trace element solution is: MgSO 4 ·7H 2 O500g / L, FeSO 4 ·7H 2 O 5g / L, MnSO 4 ·8H 2 O 5g / L, ZnSO 4 5g / L, the solvent is deionized water. ...
Embodiment 2
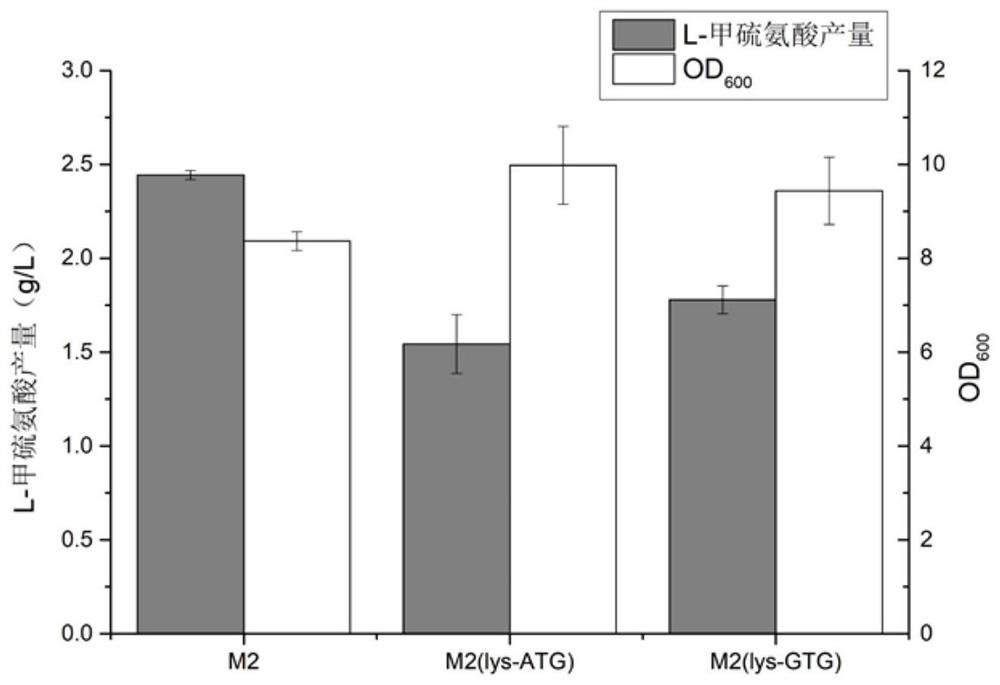
[0050] Example 2: Construction of effective bacterial strain E.coli W3110 M2-lysA-ATG / pAm and its shake flask fermentation E.coliW3110 M2 / pAm was used as the starting strain, using CRISPR-Cas9-mediated gene editing technology (Yu Jiang et al.2015Multigene Editing in the Eshcrichia coli Genome via the CRISPR-Cas9System.Applied Environmental Microbiology.81:2506-2514), in situ complementation of the lysA gene on the genome, the specific operations are as follows:
[0051] (1) Construction of pTarget-lysA plasmid: Use pTarget F plasmid (Addgene Plasmid#62226) as template, lysA-PAM-F / lysA-PAM-R in Table 2 as primers for PCR amplification, and the PCR product is incubated at 37°C with DpnⅠ Digested for 3 hours, then transformed into E.coli DH5α, inoculated on LB plates containing 50mg / L spectinomycin, cultured at 37°C for 12 hours, screened the colonies that could grow, and sequenced to verify that the correct pTarget-lysA plasmid was obtained for subsequent connection with Donor D...
Embodiment 3
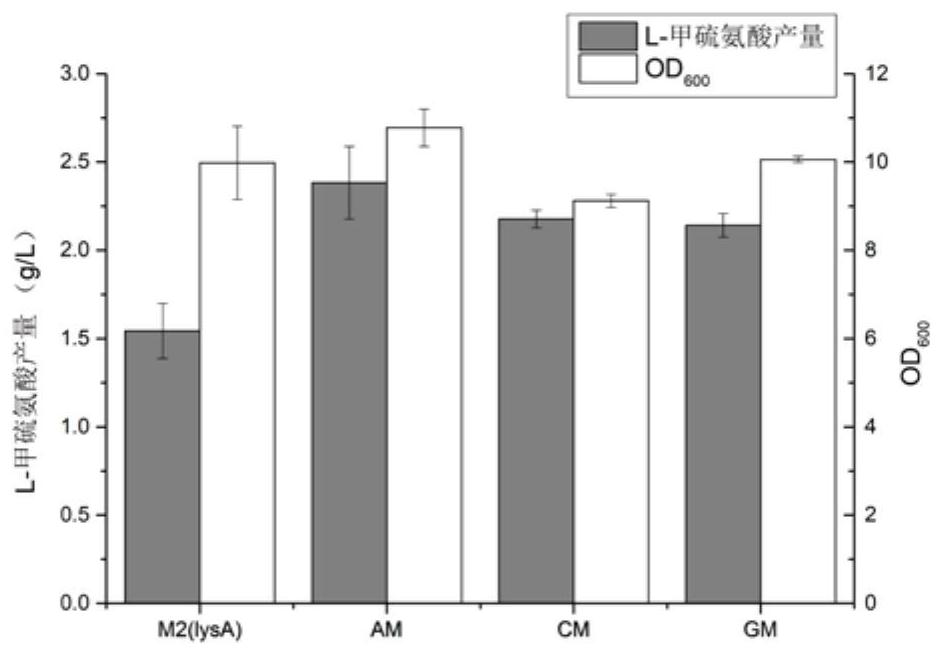
[0061] Embodiment 3: Construction effective strain E.coli W3110 M2-PfliA-lysA / pAm and shake flask fermentation thereof
[0062] (1) Construction of pTarget-lysA-2 plasmid: use pTarget F plasmid (Addgene Plasmid#62226) as template, use lysA-PAM-F-2 / lysA-PAM-R-2 in Table 2 as primers for PCR amplification, PCR The product was incubated and digested by DpnI at 37°C for 3 hours, then transformed into E.coli DH5α, inoculated on LB plates containing 50 mg / L spectinomycin, cultured at 37°C for 12 hours, and the colonies capable of growth were screened, and the correct pTarget-lysA was obtained through sequencing verification -2 plasmids for subsequent ligation of DonorDNA.
[0063] (2) Construction of pTD-lysA-2 plasmid: using the E.coli W3110 genome as a template, L-fliA-up-F, L-fliA-up-R, L-fliA-down-F, L-fliA-down- R is the primer to obtain the upstream and downstream PCR products of the lysA gene regulated by the PfliA promoter. After the Clean up kit is recovered, the fusion PC...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com