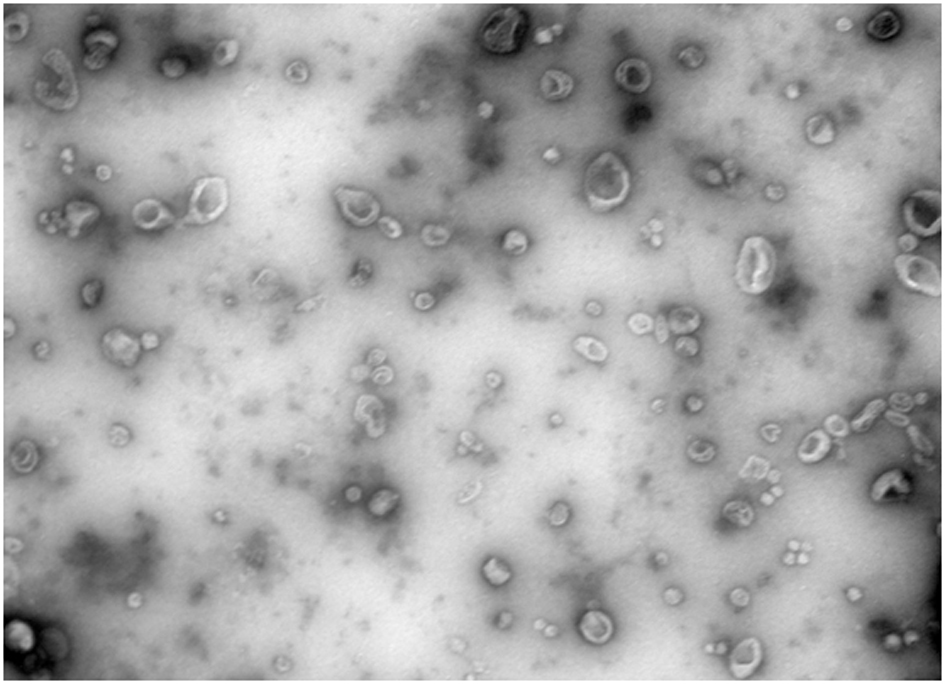
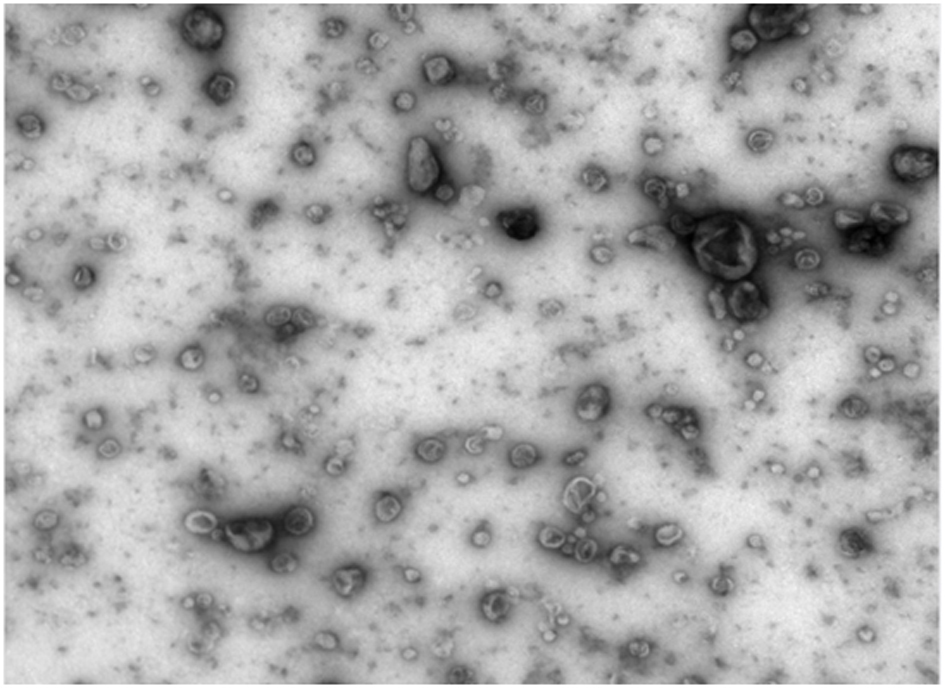
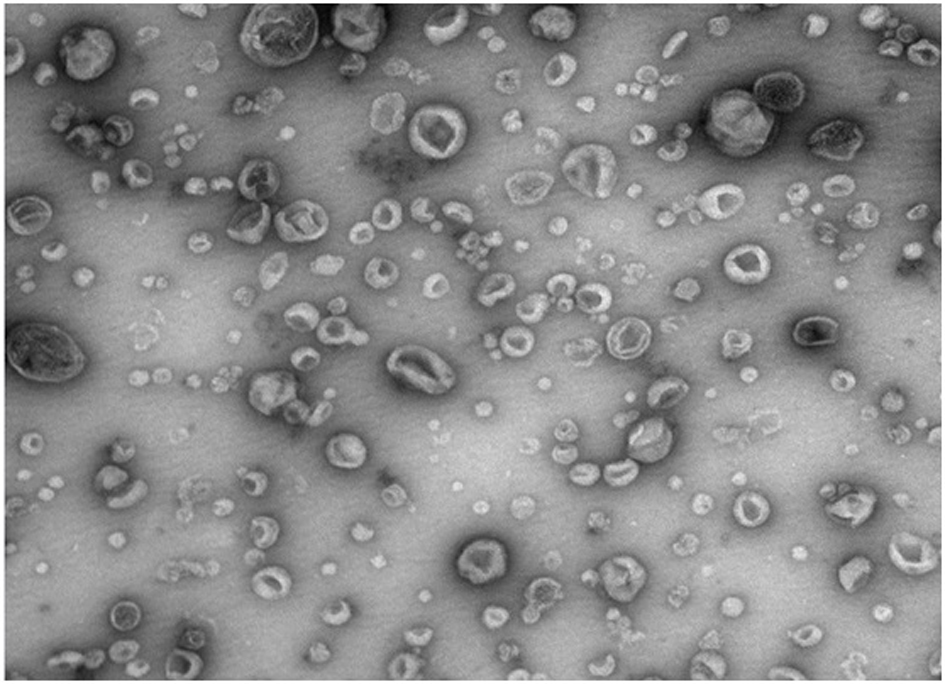
Exosome separation and purification method
A technology for separation, purification and exosomes, applied in the biological field, can solve the problems of complex operation, large influence on type, quantity and quality, and less processing volume, and achieve the effect of simplifying separation steps, shortening purification cycle, and high recovery rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] step one
[0077] 1.1 Acid precipitation
[0078] Take 2L of milk and adjust the pH to 4.6 with hydrochloric acid. During the pH adjustment, use a magnetic stirrer to stir at room temperature, and then let it stand for acid precipitation for 60min.
[0079] 1.2 Sample clarification
[0080] The acid-precipitated sample was placed in a centrifuge cup, centrifuged for 30 min under a centrifugal force of 3500 g using a centrifuge, and the centrifuged supernatant was collected. Or use a bag filter with a cut-off pore size of 10 μm by Cobaxter, and the filtration rate is 50 ml / min, and take the supernatant solution after filtration.
[0081] 1.3 Deep filtering
[0082] The centrifuged or filtered supernatant solution was further clarified and filtered using a depth filter with a cut-off pore size of 0.5-10 μm, and the filtration rate was 100L / m 2 / h to obtain a supernatant solution.
[0083] Step 2
[0084] 2.1 Buffer solution preparation
[0085] a. Prepare 0.5M NaOH...
Embodiment 2
[0126] step one
[0127] 1.1 Acid precipitation
[0128] Take 2L of milk and adjust the pH to 4.6 with hydrochloric acid. During the pH adjustment, use a magnetic stirrer to stir at room temperature, and then let it stand for acid precipitation for 60min.
[0129] 1.2 Sample clarification
[0130] The acid-precipitated samples were placed in a centrifuge cup, centrifuged for 30 min under a centrifugal force of 3500 g using a centrifuge, and the centrifuged supernatant was taken. Or use a bag filter with a cut-off pore size of 10 μm by Cobaxter to filter, and the filtration rate is 50 ml / min, and take the supernatant solution after filtration.
[0131] 1.3 Deep filtering
[0132] The centrifuged or filtered supernatant solution is further clarified and filtered using a depth filter with a cut-off pore size of 0.5-10 μm, and the filtration rate is 100 L / m2 / h to obtain a supernatant solution.
[0133] Step 2
[0134] 2.1 Buffer solution preparation
[0135] a. Prepare 0.5M ...
Embodiment 3
[0176] step one
[0177] 1.1 Acid precipitation
[0178] Take 2 L of milk and adjust the pH to 4.6 with hydrochloric acid. During the pH adjustment, use a magnetic stirrer to stir at room temperature, and then let it stand for 60 minutes for acid precipitation.
[0179] 1.2 Sample clarification
[0180] The acid-precipitated sample was placed in a centrifuge cup, centrifuged for 30 min under a centrifugal force of 3500 g using a centrifuge, and the centrifuged supernatant was taken. Or use a bag filter with a cut-off pore size of 10 μm by Cobaxter, and the filtration rate is 50 ml / min, and take the supernatant solution after filtration.
[0181] 1.3 Deep filtering
[0182] The centrifuged or filtered supernatant solution was further clarified and filtered using a depth filter with a cut-off pore size of 0.5-10 μm, and the filtration rate was 100L / m 2 / h to obtain a supernatant solution.
[0183] Step 2
[0184] 2.1 Buffer solution preparation
[0185] a. Prepare 0.5M Na...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com