Ternary electrolyte containing saturated heterocycles and preparation and application thereof
A technology of electrolytes and heterocycles, applied in the preparation of sugar derivatives, compounds containing elements of group 3/13 of the periodic table, circuits, etc., can solve problems such as correlation or deducibility uncertainty, and achieve electrochemical performance Improved, widened electrochemical window, outstanding structural effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Example 1: Raw materials
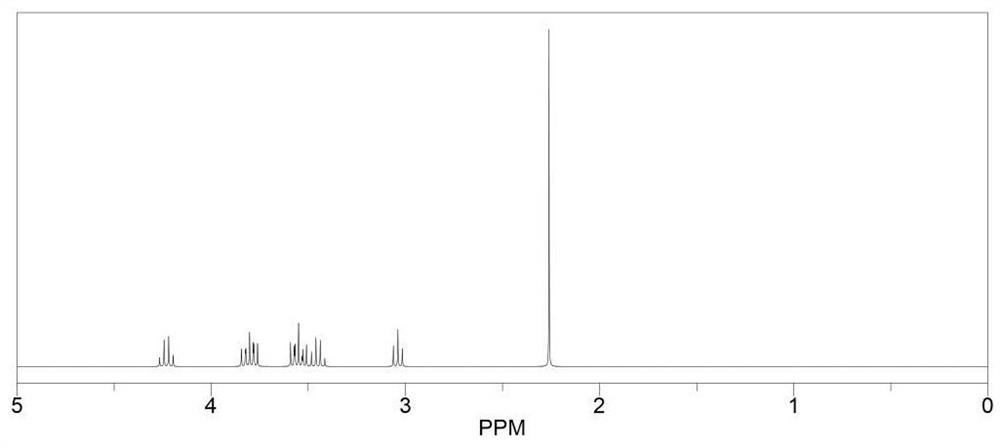
[0073] Preparation method: under nitrogen atmosphere, mix 0.01 mol of raw material and boron trifluoride tetrahydrofuran complex (4.19 g, 0.03 mol) in 15 ml of ethylene glycol dimethyl ether, and react at room temperature for 12 hours. The obtained mixed solution was dried under reduced pressure under the conditions of 40° C. and a vacuum degree of about -0.1 MPa to remove the solvent to obtain an intermediate. Lithium ethoxide (1.56g, 0.03mol) was dissolved in 10ml of ethanol and slowly added to the intermediate, and the reaction was stirred at 45°C for 8 hours, and the resulting mixture was dried under reduced pressure at 40°C and a vacuum of about -0.1MPa. , the obtained solid was washed three times with n-butyl ether, filtered and dried to obtain the product M1. The yield was 88%, NMR as figure 1 shown.
Embodiment 2
[0074] Example 2: Raw materials
[0075] Preparation method: under argon atmosphere, mix 0.01 mol of raw material and boron trifluoride diethyl ether complex (4.26 g, 0.03 mol) in 15 ml of ethylene glycol dimethyl ether, and react at room temperature for 12 hours. The obtained mixed solution was dried under reduced pressure under the conditions of 30° C. and a vacuum degree of about -0.1 MPa to remove the solvent to obtain an intermediate. 18.90ml of butyllithium in hexane solution (c=1.6mol / L) was added to the intermediate, and the reaction was stirred at room temperature for 6 hours, and the resulting mixed solution was dried under reduced pressure at 40°C and a vacuum degree of about -0.1MPa. , the obtained crude product was washed three times with cyclohexane, filtered and dried to obtain product M2, wherein Q is OBF 3 Li. The yield was 89%.
Embodiment 3
[0076] Example 3: Raw materials
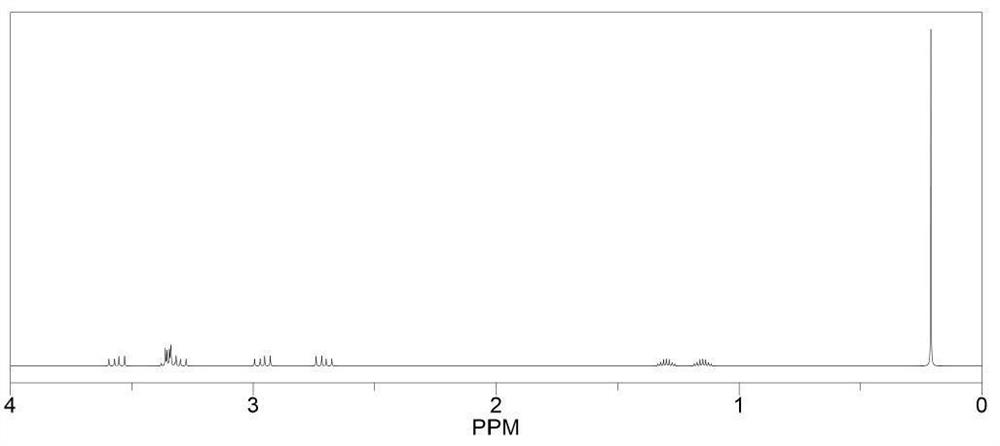
[0077] Preparation method: under nitrogen atmosphere, mix 0.01 mol of raw material and lithium methoxide (1.14 g, 0.03 mol) with 20 ml of methanol, and react at room temperature for 8 hours. The obtained mixed solution was dried under reduced pressure under the conditions of 40° C. and a vacuum degree of about -0.1 MPa to remove the solvent to obtain an intermediate. The boron trifluoride tetrahydrofuran complex (4.19g, 0.03mol) and 10ml of THF were added to the intermediate, and the reaction was stirred at room temperature for 6 hours, and the resulting mixed solution was decompressed at 40°C and a vacuum of about -0.1MPa. After drying, the resulting solid was washed three times with isopropyl ether, filtered and dried to give the product M3. Yield 79%, NMR as figure 2 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com