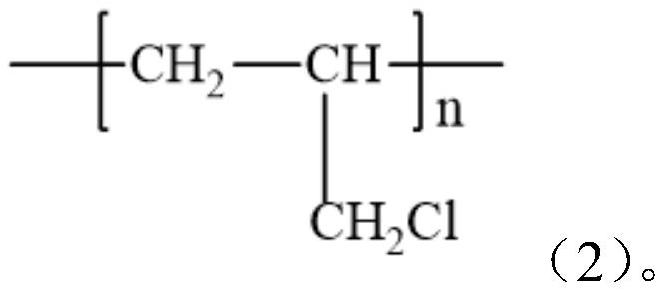Chlorohydrin ball loaded amine substance modified heteropolyacid catalyst as well as preparation and application of chlorohydrin ball loaded amine substance modified heteropolyacid catalyst
A technology of amine substances and heteropolyacids, which is applied in the application field of chlorine ball-loaded amine substances modified heteropolyacid catalysts and its preparation, lauric acid and glycerol esterification, and can solve the problem of long catalyst preparation time and easy Soluble in polar solvents, small specific surface area, etc., to solve severe corrosion equipment, low preparation cost, simple separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] In order to solve the problems of serious corrosion of equipment, pollution of the environment, and complicated post-treatment of common catalysts, the application has the advantages of simple catalyst preparation, low preparation cost, and at the same time, it is loaded on a carrier, which is convenient for the separation and purification of the catalyst after the reaction, and proposes a chlorine ball supported The preparation method of amine substance modified heteropolyacid catalyst specifically comprises the following steps:
[0049] (1) Using chlorine balls and amines as raw materials, using acetonitrile as solvent, reacting in an ultrasonic synthesizer for a period of time, cooling to room temperature for filtration, and washing with ethyl acetate, 0.1mol / L HCl, water and methanol for many times , and then vacuum-dried to obtain an amine-modified chlorine ball ultrasonic synthesizer for use. In the step, the ultrasonic reaction temperature is 60-120° C., the ultr...
Embodiment 1
[0054] The present embodiment takes chlorine ball-piperazine-silicotungstic acid as an example, and the preparation method of the catalyst is as follows:
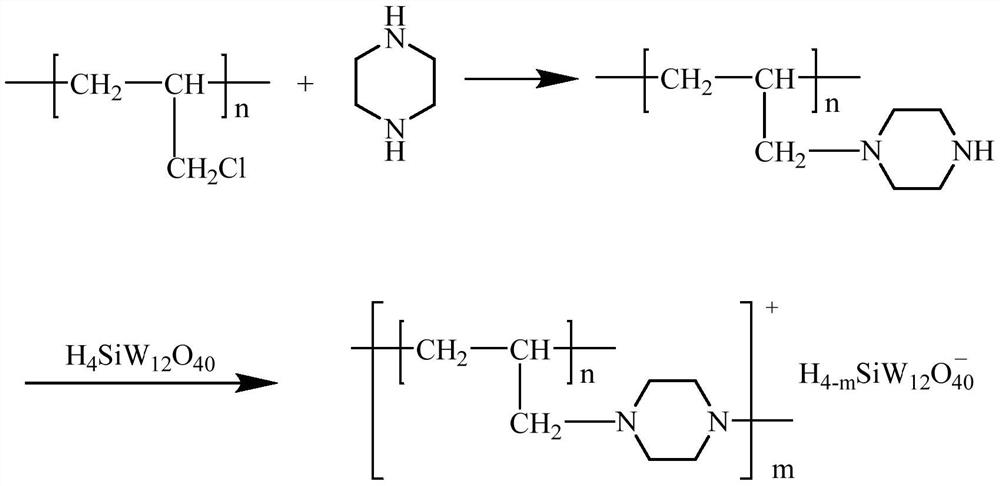
[0055](1) Using chlorine ball and piperazine as raw materials, using acetonitrile as solvent, under the conditions of reaction temperature of 80°C and ultrasonic power of 500W, placed in an ultrasonic synthesizer to react for 2.5h, cooled to room temperature after the reaction, and filtered with acetic acid. Ethyl ester, 0.1mol / L HCl, water and methanol were washed for several times, and then vacuum-dried to obtain piperazine-modified chlorine spheres, and the substance ratio of chlorine spheres to piperazine was 1:1.2.
[0056] (2) The piperazine-modified chlorine ball and silicotungstic acid were placed in a reaction vessel and reacted in an ultrasonic synthesizer. The reaction temperature was 80°C and the ultrasonic power was 500W. After vacuum drying treatment, piperazine modified chlorine spheres supported silicotungst...
Embodiment 2
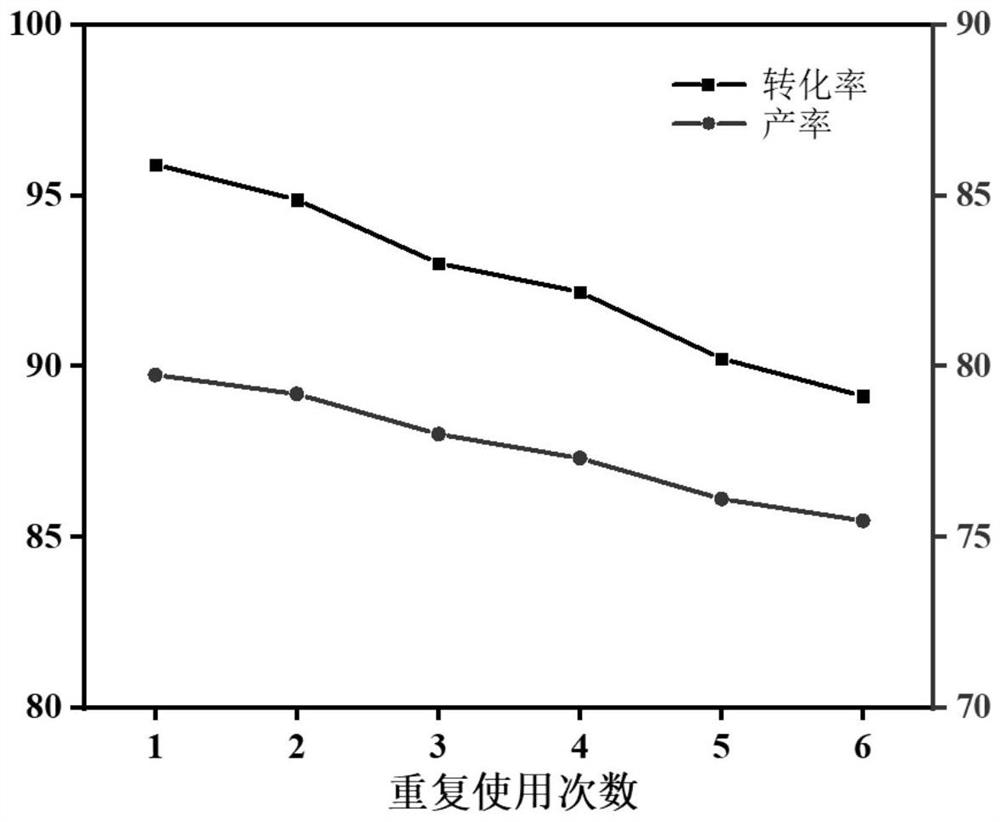
[0065] In order to verify the catalytic effect of different reaction time catalysts, in the present example, in the 100mL there-necked flask equipped with the reflux condenser, add lauric acid 10g, glycerol 23g, catalyzer 0.4g (prepared in Example 1) successively, and place it in 150 The reaction was heated in an oil bath. The reaction product was taken every half hour (one hour after 3h), and the reaction product was analyzed by gas chromatography for its conversion rate and yield. The reaction results of monoglyceryl laurate obtained by changing the reaction time are shown in Table 1.
[0066] Table 1: The reaction results of monoglyceryl laurate obtained at different reaction times.
[0067]
[0068]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com