Benzimidazopyrazine-3-carboxamide targeting A2A and tumor immune function thereof
A tumor-targeting technology, applied in the field of medicine, can solve problems such as benefiting patients, and achieve the effect of enhancing effect, inhibiting cAMP accumulation, and enhancing killing effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 1-Amino-N-(pyridin-2-ylmethyl)benzo[4,5]imidazo[1,2-a]pyrazine-3-carboxamide small molecule compound (LDH-E- 4) Preparation
[0037] The structure of LDH-E-4 is shown below:
[0038]
[0039] The preparation of this compound comprises the following steps:
[0040] (1) preparation of compound shown in formula 3:
[0041]
[0042] According to the above reaction formula, the compound shown in formula 1 (1,2-phenylenediamine, 3.2 g, 30 mmol) and 50 mL of acetic acid (AcOH) were added to the reaction flask at room temperature, and the starting materials were slowly added after cooling in an ice bath, That is, the compound represented by formula 2 (methyl 2,2,2-trichloroiminoacetate, 4 mL, 30 mmol) was added and stirred at room temperature for 2 hours, and TLC showed that the reaction was completed. After the reaction, the reactant was filtered, and the obtained filter cake was washed with water (3×25 mL) and dried under vacuum to obtain the compound repre...
Embodiment 2
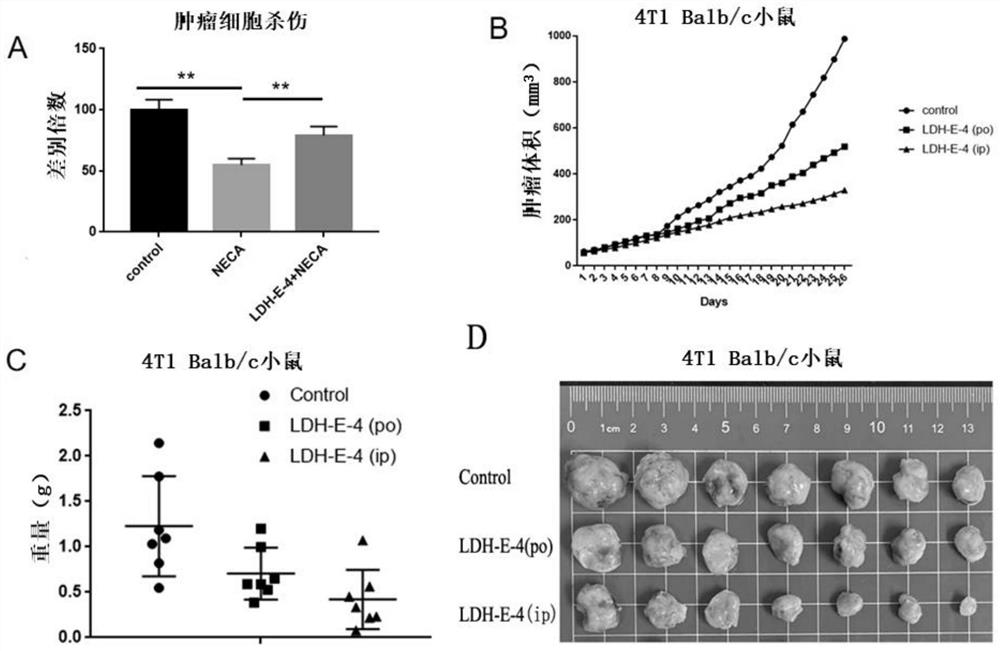
[0077] Example 2 LDH-E-4 specifically targets A 2A Research on the characteristics of R
[0078] (1) Cell culture
[0079] The A2A-HEK293 and A1-HEK293 (HEK293 cells used in this experiment were purchased from ATCC, and the construction of the overexpressed cell line is based on "Borodovsky, A., et al., Small molecule AZD4635 inhibitor of A2ARsignaling rescues immune cell function including CD103+dendritic" cellsenhancing anti-tumor immunity. Journal for ImmunoTherapy of Cancer, 2020.8(2):p.e000417."), MBA-MD-231 (purchased from ATCC) cells were cultured in cells containing 10% fetal bovine serum and 1% double antibody (penicillin and Streptomycin) in DMEM medium, 4T1 and PBMC cells were cultured in RPIM-1640 medium containing 10% fetal bovine serum and 1% double antibody (penicillin and streptomycin), and the above cells were placed at 37°C, 5%CO 2 cultured in a cell incubator.
[0080] (2) Compound pair A 2A R and A 1 Affinity testing for R
[0081] 1) Preparation of ...
Embodiment 3
[0107] Example 3 Study on the characteristic of LDH-E-4 inhibiting the accumulation of cAMP
[0108] (1) Compound and buffer configuration
[0109] Assay buffer was prepared containing 1 x HBSS (Sigma), 0.1% BSA (Perkin Elmer), 20 mM HEPES (Gibco) and 100 nM IBMX (Sigma). Use Simulationbuffer to configure 8× test compound stock solutions (10 -4 ,10 -5 ,10 -6 , 10 -7 ,10 -8 ,10 -9 ,10 -10 M) and 8x CGS21680 stock solution (50 nM). Prepare 20× cAMP-d2 and 20× anti-cAMP-Eu3+ detection reagent solutions using Lysis & Detection Buffer lysis buffer.
[0110] (2) HTRF method to test intracellular cAMP content
[0111] The HEK293-A 2A Cells were seeded in 384-well plates containing 38,000 cells per well and 15 μL of assay buffer. 2.5 μL of test compound solutions were added to designated wells of the above 384-well plate and incubated at 37°C for 10 minutes. Then, 2.5 μL of CGS21680 stock solution was added to the 384-well plate, and incubated at 37° C. for another 30 minu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com