Novel asymmetric viologen compound as well as preparation method and application thereof
An asymmetric and compound technology, applied in the field of new asymmetric viologen compounds and their preparation, can solve the problems of reducing the dynamic stability of electrochromic devices, achieve good electron accepting ability, realize pleochroism, redox state rich effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Tpy-Vio-1-X - (X 1 =Cl,X 2 =I) preparation
[0047]
[0048] (1) Preparation of compound 5: 1-chloro-2,4-dinitrobenzene (300 mg, 1.49 mmol) and 4,4'-bipyridine (348 mg, 2.20 mmol) were refluxed in anhydrous acetonitrile for 48 h, After the reaction, cooled to room temperature, filtered, the filtrate was spin-dried and washed three times with acetone and diethyl ether, then filtered and dried in vacuo to obtain compound 5. Yield: 70%.
[0049] 1 H NMR (400MHz, D 2 O)δ(ppm):9.30(d,J=2.5Hz,1H),9.16(d,J=7.1Hz,2H),8.84(dd,J=8.7,2.5Hz,1H),8.76-8.73(m , 2H), 8.59 (d, J=7.1Hz, 2H), 8.18 (d, J=8.7Hz, 1H), 7.95-7.91 (m, 2H).
[0050] (2) Preparation of compound 3: Compound 1 (500 mg, 3.31 mmol) was completely dissolved in methanol, then compound 2 (800 mg, 6.62 mmol) was added, 15 mL of 15% KOH aqueous solution was added, 15 mL of ammonia water was added, and the mixture was stirred at 25° C. for 3 After the reaction was completed, a large amount of black so...
Embodiment 2
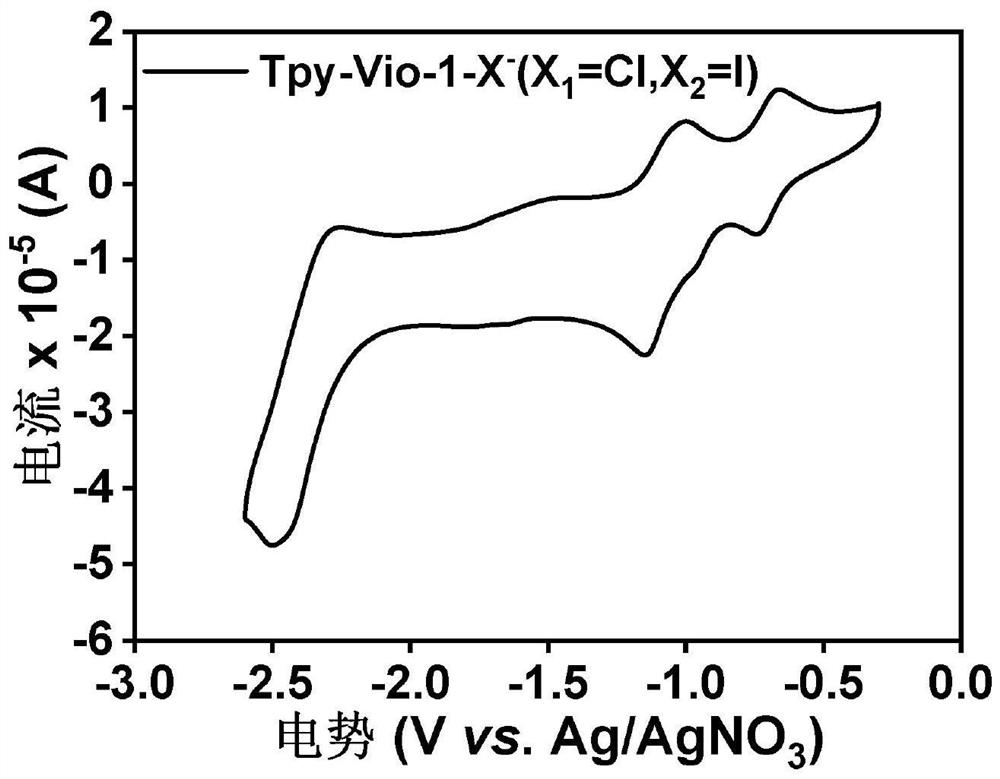
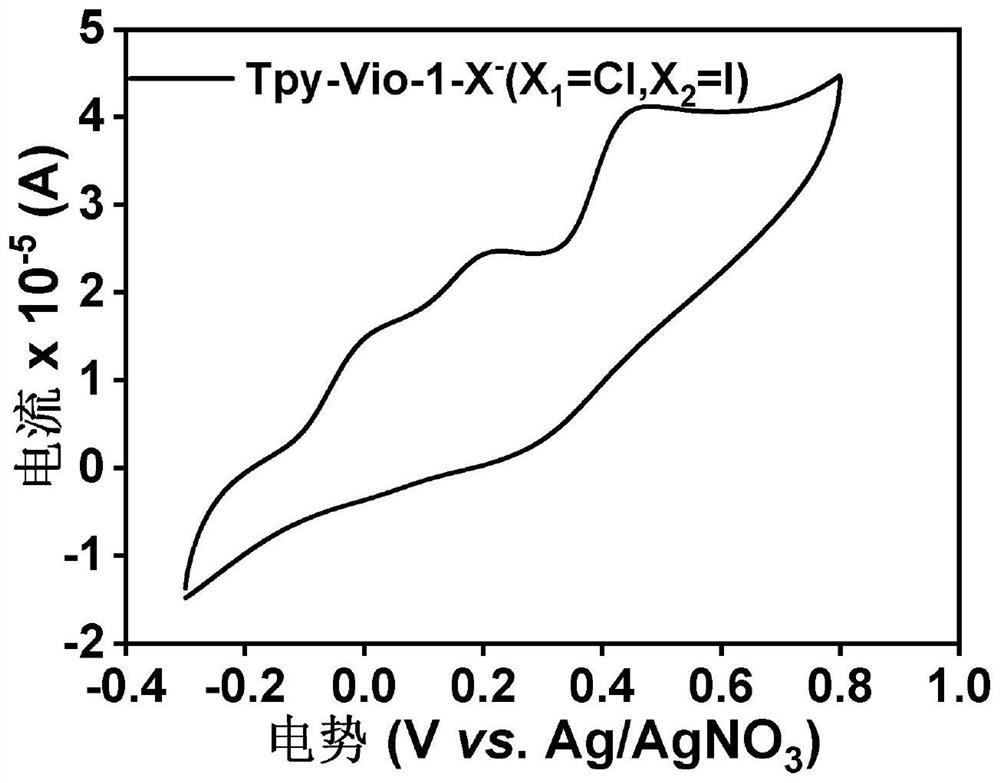
[0059] Example 2: Tpy-Vio-1-X - (X 1 =Cl,X 2 =I) Cyclic Voltammetry Test
[0060] Tpy-Vio-1-X - (X 1 =Cl,X 2 =1) The cyclic voltammetry test adopts a three-electrode system, the counter electrode is a platinum wire electrode, the working electrode is a palladium carbon electrode, and the reference electrode is Ag / AgNO 3 . Tetrabutylammonium hexafluorophosphate in DMF (0.1 M) was used as electrolyte. Scanning speed is 100mV·s -1 .
[0061] Tpy-Vio-1-X - (X 1 =Cl,X 2 The cyclic voltammogram of =I) is shown in Figure 1. Depend on Figure 1a It can be seen that this compound has three pairs of reversible redox peaks at the negative level, of which the two pairs of reversible redox peaks at the low potential of the negative potential correspond to the reduction potential of the viologen double cation to gain one electron and two electrons, close to the high potential. A pair of reversible redox peaks at the potential corresponding to the gain and loss of electrons from...
Embodiment 3
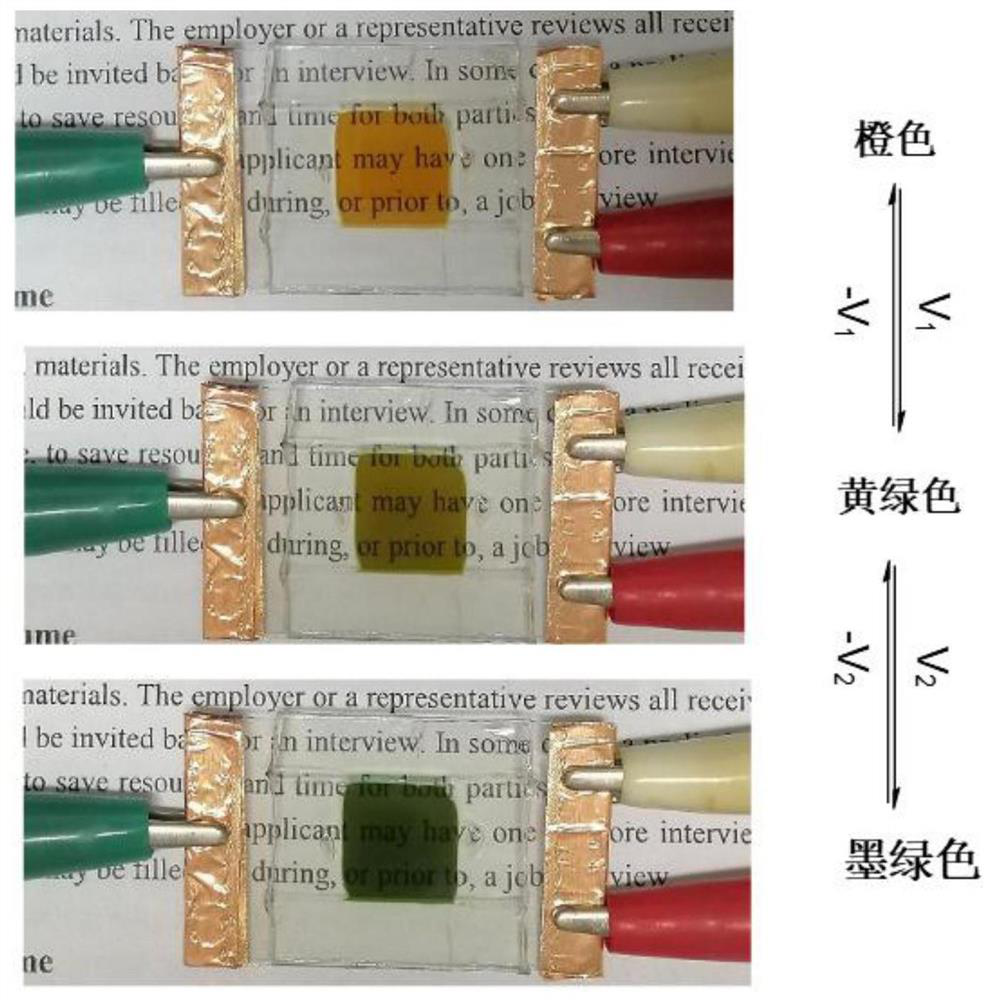
[0062] Example 3: Using Tpy-Vio-1-X - (X 1 =Cl,X 2 =I) The color change of the fabricated device under electrical stimulation
[0063] Tpy-Vio-1-X - (X 1 =Cl,X 2 =I) The cyclic voltammogram of the device is as follows figure 2 shown. It can be seen from the figure that this compound is an electroactive material doped with a suitable electrolyte to prepare an electrochromic device. When no voltage is applied, it displays orange, and a preliminary voltage V is applied. 1= After -1.2V, the color of the device changes to yellow-green, and then continue to apply the voltage V 2= After -1.8v, the color of the device changes to dark green.
PUM
| Property | Measurement | Unit |
|---|---|---|
| transmittivity | aaaaa | aaaaa |
| transmittivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com