Enzyme co-expression system and its application in the synthesis of sialic acid
An expression vector and recombinase technology, applied in the field of biochemistry, can solve the problems that the sialic acid reaction efficiency does not meet the requirements of industrial production, the second-step reaction yield is low, the sialic acid yield is low, etc. The effect of improving catalytic activity, improving yield, and improving balance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
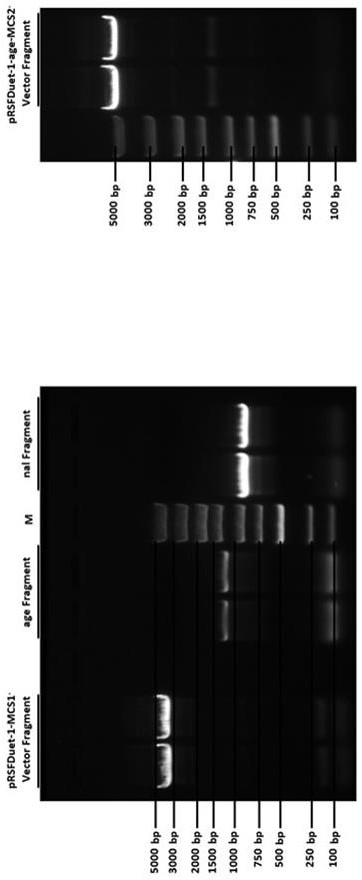
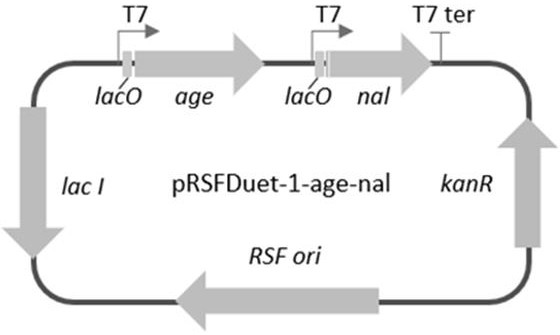
[0060] Example 1 Construction of plasmid vector pRSFDuet-1-age-nal by homologous recombination
[0061] (1) Construction of AGE, NAL target genes
[0062] The target genes AGE and NAL were synthesized by gene synthesis, and the gene sequences are shown in Table 1. The synthesized gene was ligated and inserted into the pET28a expression vector, using two sites of NdeI and XhoI restriction enzymes to form pET28a-age and pET28a-nal original plasmids.
[0063] Table 1 Gene sequence
[0064]
[0065]
[0066]
[0067] Among them, three mutation points, underlined and bold indicate the mutated gene sequence.
[0068] (2) Design primers
[0069] Through PCR amplification, four plasmid fragments required for homologous recombination were obtained. See Table 2 for primer names and sequences:
[0070] Table 2 Primer names and sequences
[0071]
[0072]
[0073] The four PCR reactions were, using pRSFDuet-1 as a template, amplified w...
Embodiment 2
[0076] Example 2 Culture expression of sialic acid synthase
[0077] (1) Plasmid transformation
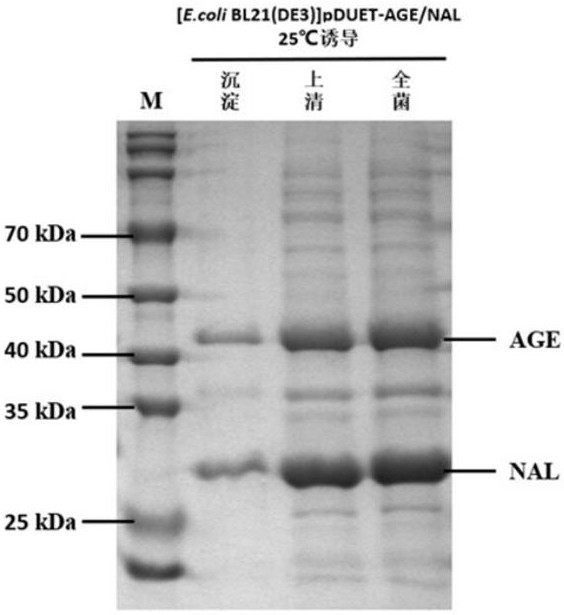
[0078] The complete plasmid pRSFDuet-1-age-nal obtained in Example 1 was transformed into competent cells large intestine B121(DE3) by heat shock. Plate culture was performed, and finally single clones were picked for improved LB liquid culture. When the cell OD reached about 0.6, about 1 mL of seed was raised, and the remaining cell solution was added with 0.5 mM isopropyl-β-D-thiogalactopyranoside (IPTG) to induce protein expression at 25 °C for 10 hours, and finally collected by high-speed centrifugation. Cells (6000 rpm, 15 min) to obtain wet cells. Take a small amount of cells and mix them with Tris-HCl buffer (50 mM, pH 8.0), and then use the freeze-thaw method to break the cells. -Determination of protein expression by polyacrylamide gel electrophoresis (SDS-PAGE), please see the protein expression results image 3 .
[0079] (2) Expanded training
[0080] ...
Embodiment 3
[0081] Example 3 Preparation of crude enzyme solution
[0082] Take the Escherichia coli cells after confirming the correct protein expression in Example 2, resuspend them in an appropriate volume of buffer (50 mM Tris-HCl, pH=7.5), use high pressure homogenization to break the cell wall, and centrifuge at high speed (12000 rpm, 10 min) to obtain the supernatant containing the two enzymes, which is the crude enzyme solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com