Phosphonic acid functionalized polybenzimidazole as well as preparation method and application thereof
A technology of benzimidazole and functionalization, which is applied in the field of high-temperature proton exchange membranes, can solve the problems of low proton conductivity of proton exchange membranes, the inability to realize accurate and high-efficiency, and difficult control of atmosphere conditions, and achieve excellent proton conductivity. , good dissolution and film-forming properties, good thermal stability and mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
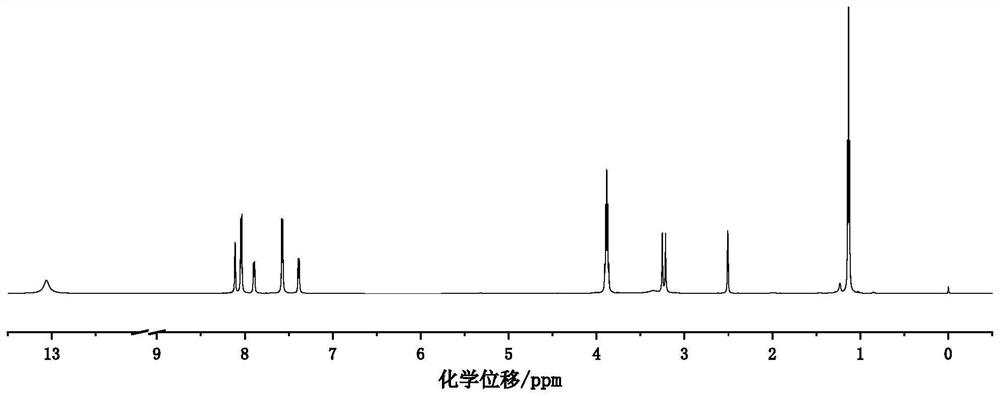
[0044] Such as figure 1 As shown, in a dry 1000ml three-necked flask, 28.4g (0.1mol) 2-methyl-[1,1'-biphenyl]-4,4'-dicarboxylic acid dimethyl ester, 17.8g (0.1mol ) N-bromosuccinimide and 0.82g (0.05mol) of azobisisobutyronitrile were dissolved in 400mL of carbon tetrachloride, and the temperature was raised to 83°C under nitrogen protection and refluxed for 3h. After the reaction was complete, the solvent was removed by a rotary evaporator at 45°C, the yellow crude product was recrystallized twice with isopropanol, and dried under vacuum at 45°C to obtain a white powder, which was 2-(bromomethyl)-[1, Dimethyl 1'-biphenyl]-4,4'-dicarboxylate. Yield: 63%.
[0045] In a dry 500ml three-necked flask, 20.1g (0.055mol) 2-(bromomethyl)-[1,1'-biphenyl]-4,4'-dicarboxylic acid dimethyl ester, 6.6g (0.03mol ) zinc bromide and 30 g (0.181 mol) of triethyl phosphite were dissolved in 240 ml of 1,4-dioxane, and the temperature was raised to 103° C. for 8 hours under nitrogen protection....
Embodiment 2
[0048] In a dry 1000ml three-necked flask, 28.4g (0.1mol) dimethyl-[1,1'-biphenyl]-4,4'-dicarboxylate, 17.8g (0.1mol) N- Bromosuccinimide and 0.82g (0.05mol) of azobisisobutyronitrile were dissolved in 300mL of carbon tetrachloride, and the temperature was raised to 75°C under nitrogen protection and refluxed for 2h. After the reaction was complete, the solvent was removed by a rotary evaporator at 45°C, the yellow crude product was recrystallized twice with isopropanol, and dried under vacuum at 45°C to obtain a white powder, which was 2-(bromomethyl)-[1, Dimethyl 1'-biphenyl]-4,4'-dicarboxylate. Yield: 59%.
[0049] In a dry 500ml three-necked flask, 20.1g (0.055mol) 2-(bromomethyl)-[1,1'-biphenyl]-4,4'-dicarboxylic acid dimethyl ester and 30g (0.181mol) Triethyl phosphite was dissolved in 240ml of 1,4-dioxane, and the temperature was raised to 103°C for 8 hours under the protection of nitrogen. When the reaction was complete, the solvent was removed by a rotary evaporato...
Embodiment 3
[0052] In a dry 250ml three-necked flask, 14.2g (0.05mol) of 2-methyl-[1,1'-biphenyl]-4,4'-dicarboxylic acid dimethyl ester, 13.4g (0.075mol) of N-bromo Substituted succinimide and 0.49g (0.03mol) of azobisisobutyronitrile were dissolved in 180mL of carbon tetrachloride, and the temperature was raised to 75°C under nitrogen protection and refluxed for 6h. After the reaction was complete, the solvent was removed by a rotary evaporator at 50°C, the yellow crude product was recrystallized twice with isopropanol, and dried under vacuum at 45°C to obtain a white powder, which was 2-(bromomethyl)-[1, Dimethyl 1'-biphenyl]-4,4'-dicarboxylate. Yield: 55%.
[0053] In a dry 500ml three-necked flask, 9.08 (0.025mol) 2-(bromomethyl)-[1,1'-biphenyl]-4,4'-dicarboxylic acid dimethyl ester and 10g (0.06mol) Triethyl phosphate was dissolved in 150ml of 1,4-dioxane, and heated to 103°C under nitrogen protection to reflux for 12h. When the reaction was complete, the solvent was removed by a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com