Preparation and structure identification method of unknown impurities of amikacin sulfate injection
A technology of amikacin sulfate and unknown impurities, applied in the field of preparation and structure identification of unknown impurities in amikacin sulfate injection, can solve the problems of uncontrollable adverse reactions, affecting safety, limiting the content of impurities, etc. Ensuring the quality and drug safety, the effect of the method is simple
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Separation and purification of unknown impurities in amikacin sulfate injection by the following steps:
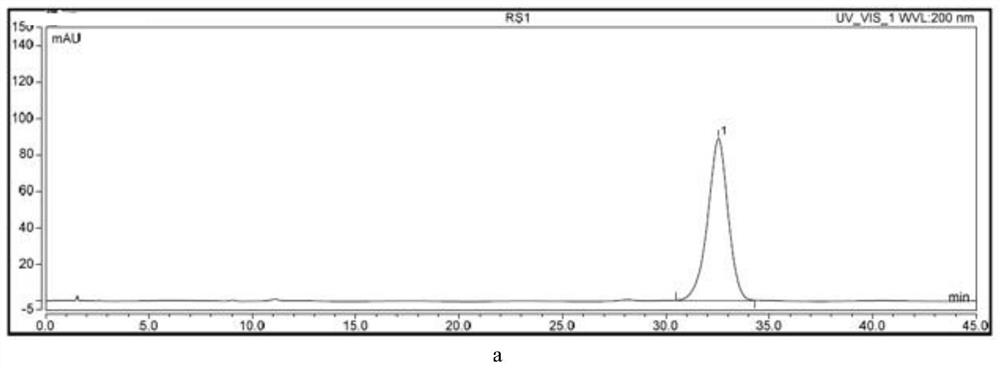
[0050] Heat and stir 80ml of Amikacin Sulfate Injection until saturated, cool and crystallize and filter, the resulting filtrate is crystallization mother liquor, concentrate to dryness under reduced pressure to obtain a solid sample containing about 2% of the unknown impurity, then add 10ml Dissolve dichloromethane, add 8g of 200 mesh silica gel, concentrate to dryness under reduced pressure, then use dry method to prepare sample, put it on silica gel column, and use petroleum ether-ethyl acetate with volume ratio of 20:1, 10:1, 0:1 to mix successively The volume of each solution was 500mL, eluted and washed into the column, and the components with Rf of 0.5 were collected, concentrated to dryness under reduced pressure to obtain 200mg of 10% crude product of the unknown impurity, dissolved in 10ml of purified water, prepared into a solution, and carried out gradien...
Embodiment 2
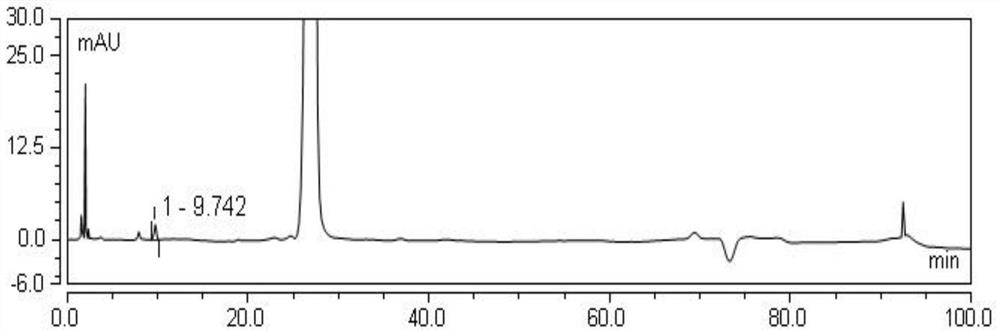
[0059] Separation and purification of this unknown impurity in amikacin sulfate injection by the following steps: measure amikacin sulfate injection 40ml until saturated under heating and stirring conditions, suction filtration after cooling and crystallization carries out solid-liquid separation, and the gained filtrate is The crystallized mother liquor was concentrated to dryness under reduced pressure to obtain a solid sample containing about 2% of the unknown impurity, then added 10ml of dichloromethane to dissolve it, added 8g of 200 mesh silica gel, concentrated to dryness under reduced pressure, and then prepared the sample by dry method and put it on a silica gel column , followed by volume ratios of 20:1, 10:1, 0:1 petroleum ether - ethyl acetate mixed solution volume 250mL elution washed column, collected components with Rf 0.5, concentrated to dryness under reduced pressure to obtain 10% 100 mg of the crude unknown impurity was dissolved in 5 ml of purified water to ...
Embodiment 3
[0062] The structure of the obtained unknown impurity was analyzed by the following steps:
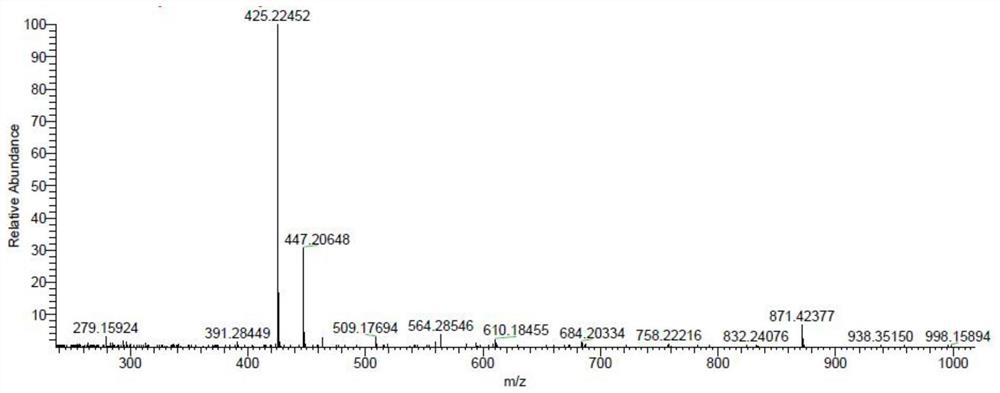
[0063] Molecular weight determination: Weigh about 2.00mg of unknown impurity sample into a 5ml volumetric flask, dissolve with purified water and dilute to the mark to prepare unknown impurity mother liquor. Remove 1ml of the unknown impurity mother solution, place it in a 10ml volumetric flask, dilute to the mark with purified water, and prepare an unknown impurity solution. LC-MS analysis conditions are preferably Agilent 1200-Themo-LTQ ORBITRAPXL, chromatographic column: Unisil 10-120 C18 Aq 10μm 4.6*250mm, mobile phase: A phase 0.1% formic acid solution, B phase acetonitrile solution, gradient elution (0 -4min, 100%A; 4-8min, 100%A→90%A; 8-10min, 90%A; 10-10.010, 90%A→100%A; 10.010-14min, 100%A), column temperature : 30°C, UV detector wavelength: 220nm, column flow rate: 0.8ml / min, injection volume: 100μl, run time 14min, ion source: ACPI, positive ion mode, fragmentation voltage...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com