Morpholine-indanone-chalcone derivative fluorescent probe as well as preparation method and application thereof
A chalcone derivative, fluorescent probe technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of low sensitivity, long response time, small Stokes shift, etc., to achieve synthesis And the effect of simple post-processing method, high fluorescence recognition performance, and wide application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
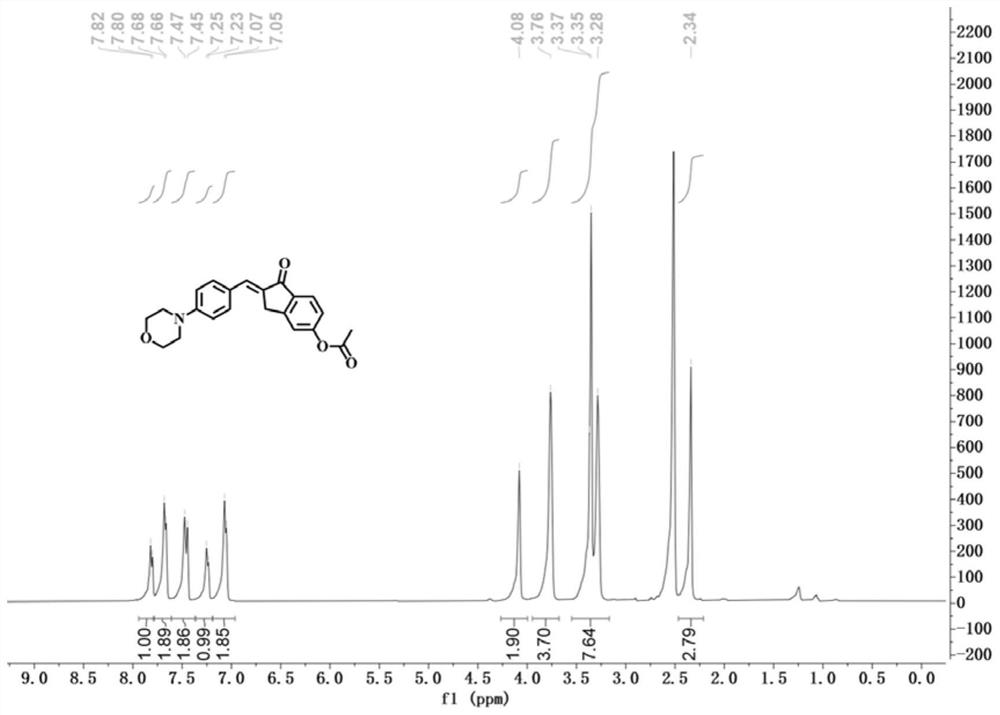
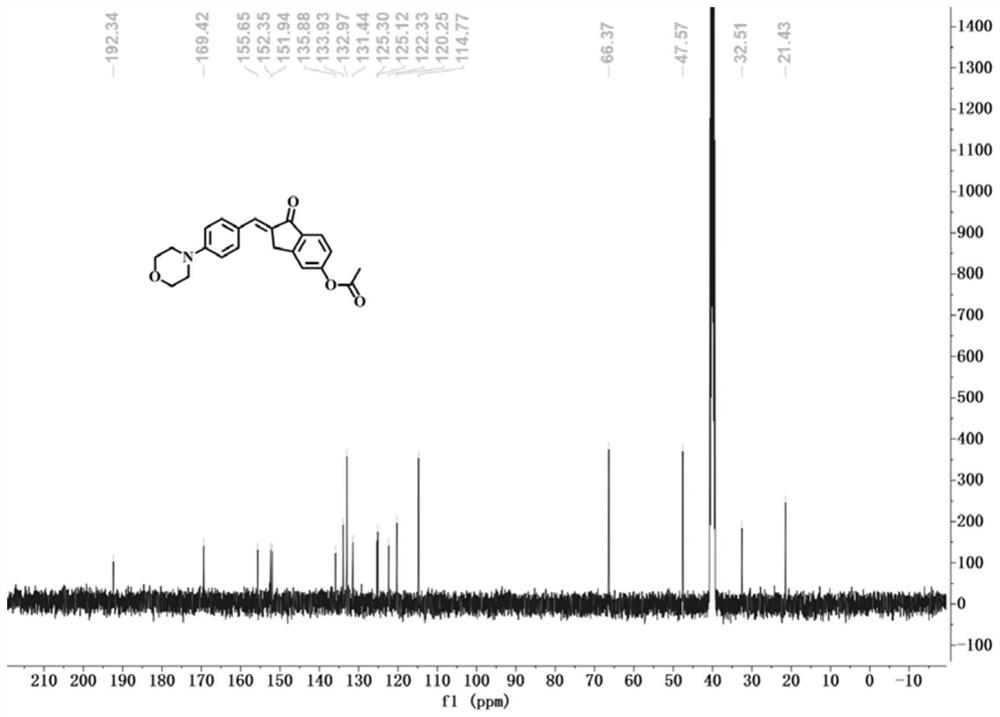
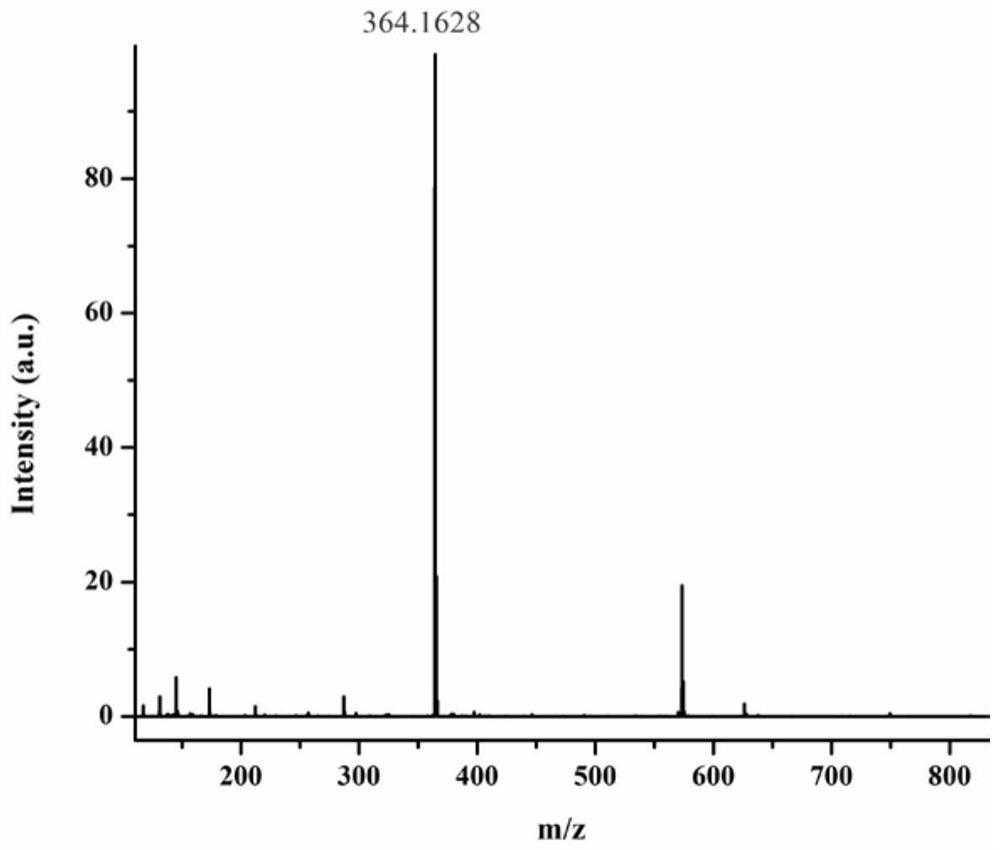
[0037] The preparation method of the morpholine-indanone-chalcone derivative fluorescent probe of this embodiment is as follows:
[0038] To a solution of 5-hydroxy-1-indanone (2mmol, 0.296g) in absolute ethanol (10mL) was added 4-(4-morpholine)benzaldehyde (2mmol, 0.382g) and sodium hydroxide (3mmol, 0.12g ); The mixture was then stirred at 50° C. for 5 h. After the reaction was completed, the solution was cooled to room temperature, acidified with hydrochloric acid to pH = 6, and evaporated under reduced pressure. The resulting solid residue was recrystallized with ethanol, filtered under reduced pressure, and dried to obtain the intermediate 5-hydroxy-2-(4-mol (Phenylbenzyllidene)-2,3-dihydro-1H-indanone. Then 5-hydroxy-2-(4-morpholino benzyl subunit)-2,3-dihydro-1H-indanone (0.14mmol, 0.0458g), acetic anhydride (0.17mmol, 16μL,) and triethyl The amine (0.42 mmol, 58 μL) was dissolved in dichloromethane, and the resulting solution was stirred at room temperature for 5 h. ...
Embodiment 2
[0044] Determination of Optical Properties of Morpholine-Indanone-Chalcone Derivatives on CEs
[0045] The morpholine-indanone-chalcone derivative prepared in Example 1 above was used as a fluorescent probe in PBS / DMSO (95 / 5, v / v, 10 mM, pH=7.4) to prepare a molar concentration of 1× 10 -5 mol / L solution, add 2×10 -4 mol / L Enzyme analytes (CEs, acetylcholinesterase, cholesterol esterase, lactoproteinase, hyaluronidase, lipase, lysozyme and polyphenol oxidase), amino acids (Ala, Arg, Asp, Cys, Hcy, Leu and Ser), redox active species (C 6 h 12 o 6 , GSH, HClO, H 2 o 2 and V c ) and other ions (Al 3+ , Ca 2+ , Fe 3+ , Mg 2+ , CO 3 2- 、HCO 3 - 、OAc - and SO 4 2- ), using a UV-Vis spectrophotometer or a fluorescence spectrometer to analyze (excitation wavelength is 400nm), the resulting fluorescence spectrum is shown in Figure 4 and Figure 5 . pass Figure 4 and Figure 5 It can be seen that the morpholine-indanone-chalcone derivatives prepared by the prese...
Embodiment 3
[0048] Detection experiment of morpholine-indanone-chalcone derivative fluorescent probe in intracellular CEs
[0049] 1 x 10 for C6 cells -5 The morpholine-indanone-chalcone derivative fluorescent probe prepared in the above-mentioned Example 1 of mol / L was incubated at 37° C. for 30 min, or with 1×10 -4 mol / L CEs inhibitor bis(4-nitrophenyl) phosphate (BNPP) was incubated for 30 min and then 1 × 10 -5 The mol / L morpholine-indanone-chalcone derivative fluorescent probe prepared in Example 1 above was incubated at 37° C. for 30 min to obtain the fluorescence imaging image of C6 cells. Specific as Figure 7 As shown, where: a is the fluorescence imaging image of the green channel of the fluorescent probe; b is the bright field image of the fluorescent probe; c is the superimposed image of the green channel and the bright field of the fluorescent probe; d is the green channel of the fluorescent probe + BNPP Fluorescence imaging image; e is the bright field image after fluores...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com