Binol-diform condensed o-aminophenol Schiff base and its synthesis method and application
A technology of ortho-aminophenol and Schiff base, which is applied in the field of Binol-diform condensed o-aminophenol Schiff base and its synthesis and application, can solve the problem that Schiff base has no ability to recognize metals, and achieve enhanced fluorescence recognition ability , the effect of excellent fluorescent performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
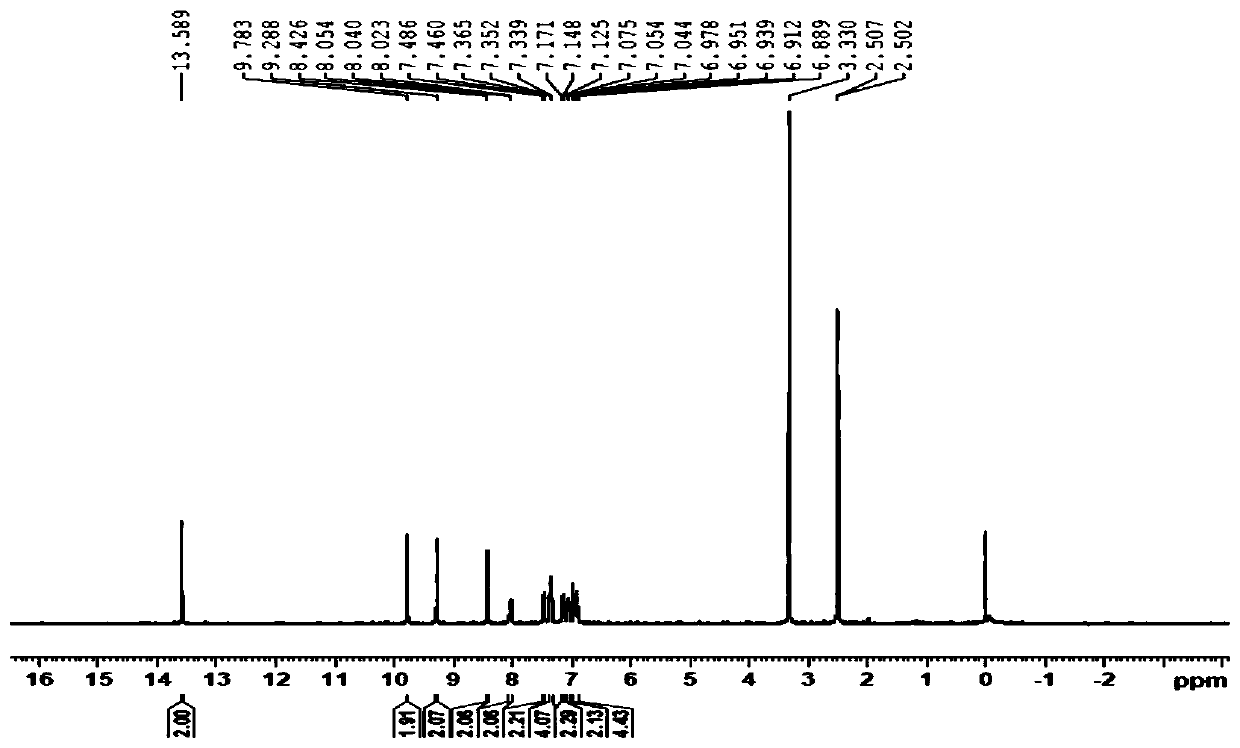
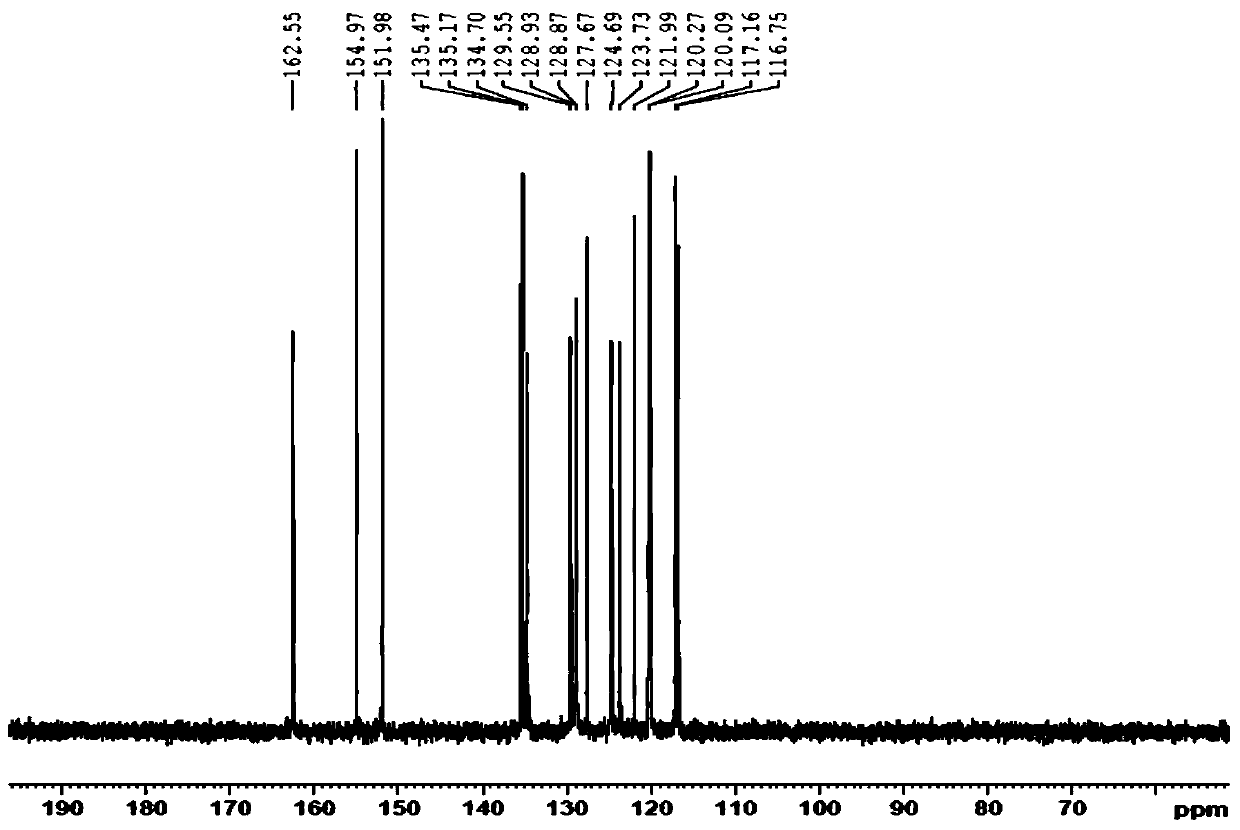
[0036] Specific embodiment 1: In this embodiment, the structural formula of the Binol-diform condensed o-aminophenol Schiff base fluorescent probe compound is as follows:
[0037]
[0038] where R is -H or -NO 2 .
specific Embodiment approach 2
[0039] Specific embodiment two: the synthesis method of Binol-diform adeno-aminophenol Schiff base of this embodiment, the steps are as follows:
[0040] 1. Dissolve o-aminophenols in an organic solvent, then add Binol-diform, and heat for 1 to 2 hours to obtain a red liquid, namely 2,2'-dihydroxy-1,1'-binaphthyl-3,3 Crude product of '-diformaldehyde-condensed o-aminophenol Schiff base;
[0041] 2. Concentrate the crude product of 2,2'-dihydroxy-1,1'-binaphthalene-3,3'-dicarbaldehyde adeno-aminophenol Schiff base, dissolve it in methanol, filter it with suction, and wash it to obtain a red solid. Vacuum drying, the obtained pure product is 2,2'-dihydroxy-1,1'-binaphthyl-3,3'-dimethylaldehyde adeno-aminophenol Schiff base; wherein in step 1, Binol-diform and o-amino The molar ratio of phenol is 1:2~3.
[0042] The Binol-diform described in Step 1 is 2,2'-dihydroxy-1,1'-bi-3,3'-dinaphthaldehyde.
specific Embodiment approach 3
[0043] Embodiment 3: This embodiment is different from Embodiment 2 in that: in the preparation step 1, the o-aminophenol is o-aminophenol or 2-amino-5-nitrophenol. Others are the same as in the second embodiment.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com