Method for preparing piperazine
A technology of piperazine and hydroxyethylpiperazine, applied in the field of preparation of piperazine, can solve the problems of low yield selectivity and high separation energy consumption, achieve high yield, improve catalytic activity, high selectivity and conversion rate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
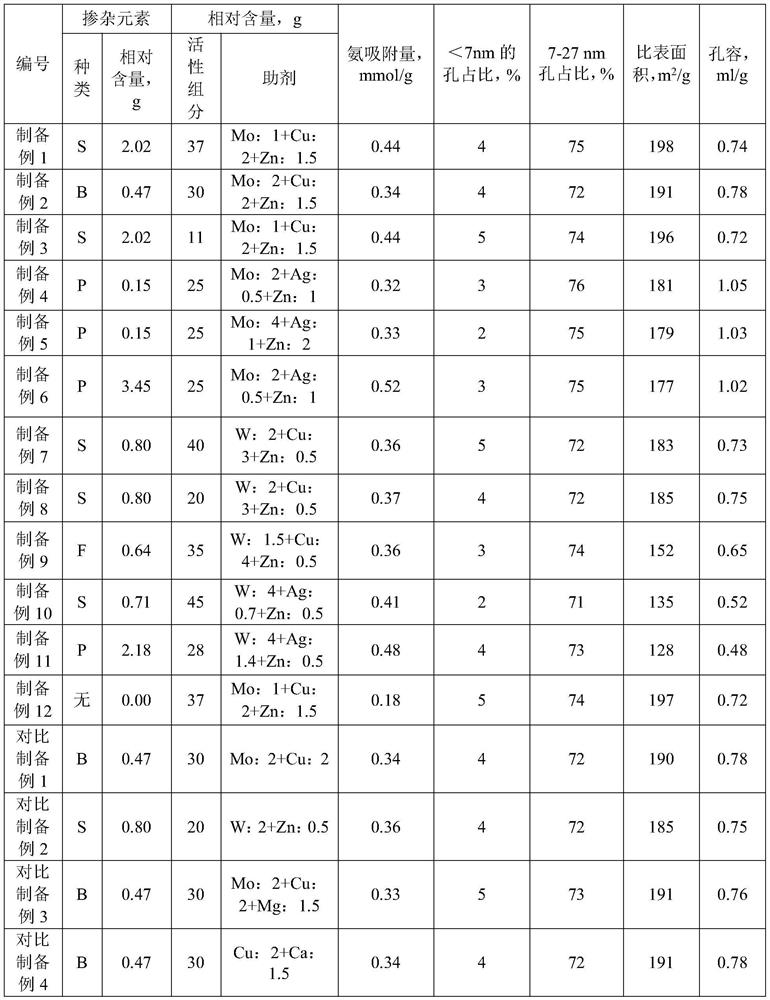
[0022] According to the present invention, the alumina precursor used in the carrier is prepared by adding silicon oxide precursor and / or molecular sieve precursor, etc., which can further greatly improve the diffusivity and pore structure stability of the catalyst after being prepared as a carrier. Therefore, according to a preferred embodiment of the present invention, the content of the alumina carrier in the carrier accounts for more than 70% by weight of the total amount of the alumina carrier and other carriers, preferably 80-97% by weight.
[0023] According to a preferred embodiment of the present invention, the content of the doping element accounts for 0.05-5% by weight of the content of the carrier, preferably 0.08-3% by weight.
[0024] According to a preferred embodiment of the present invention, the doping element in the alumina precursor of the carrier is doped with at least one of borate ions, fluoride ions, phosphate ions, sulfate ions and selenate ions. The d...
preparation example 1
[0048] Pseudoboehmite powder (specific surface area 380m 2 / g, pore volume 1.02ml / g, pseudoboehmite powder contains doping element S, relative to 100g Al 2 o 3 The calculated pseudo-boehmite powder contains 2.15g of S element; during the preparation of the pseudo-boehmite powder, SiO is added at the beginning 2 Precursor water glass (sodium silicate aqueous solution), so that after sintering, the carrier is derived from SiO 2 Precursor of SiO 2 The mass accounts for 4% of the total mass of the carrier) kneaded with dilute acid water containing 5vol% nitric acid, extruded into strips with a diameter of 5mm after kneading, cut into 4mm lengths, dried at 120°C for 8h, and then calcined at 650°C for 5h. Make the desired carrier.
[0049] 186.5g cobalt nitrate hexahydrate (industrial grade, purity 98%), 6.83g zinc nitrate hexahydrate (analytical pure) and 5.65g copper nitrate trihydrate (analytical pure) were dissolved in water into 148mL solution, which was sprayed twice This...
preparation example 2
[0051] Pseudo-boehmite powder prepared by aluminum sulfate method (specific surface area 375m 2 / g, pore volume 0.98ml / g, pseudoboehmite powder contains doping element B, relative to 100g Al 2 o 3 The calculated pseudo-boehmite powder contains 0.53g of B element; during the preparation of the pseudo-boehmite powder, SiO is added at the beginning 2 Precursor water glass (sodium silicate aqueous solution), so that after sintering, the carrier is derived from SiO 2 Precursor of SiO 2 The mass accounts for 11% of the total mass of the carrier)) kneaded with dilute acid water containing 5vol% nitric acid, extruded into a 3mm thick clover shape after kneading, dried at 100°C for 12h, and then calcined at 590°C for 8h to obtain the required carrier.
[0052] Dissolve 151.7g nickel nitrate hexahydrate (industrial grade, purity 98%), 6.83g zinc nitrate hexahydrate (analytical pure) and 5.65g copper nitrate trihydrate (analytical pure) into 156mL solution with water, and use spray d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com