Preparation method of amino-acid ester or deuterated amino-acid ester compound
A technology of ester compounds and amino acid esters, which is applied in the field of preparation of amino acid esters or deuterated amino acid esters, can solve the problems of unsatisfactory application value and market value, complex deuterated amino acid synthesis steps, and immature synthesis technology. Achieve significant social and economic benefits, the preparation method is easy to operate, and the effect of easy large-scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055]
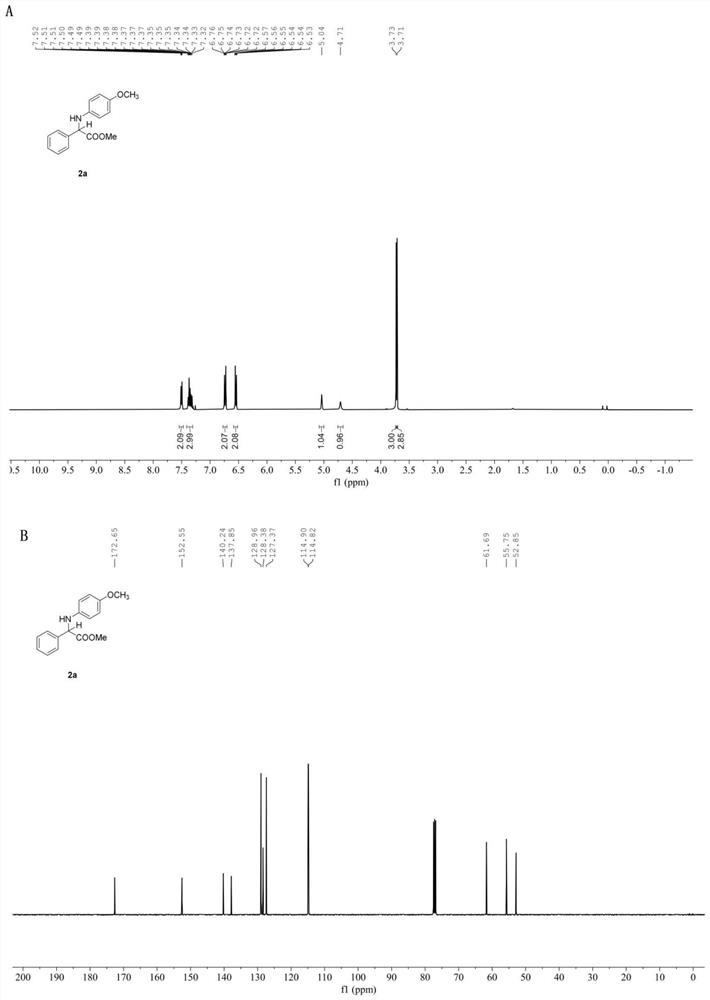
[0056] In the glove box, weigh the photosensitizer Ru(bpy) 3 Cl 2 (52mg, 2 mol%), the corresponding imidate 1a (4mmol), add acetonitrile (20.0 mL), water (720mL, 40mmol), phenylsilane (1731mg, 16 mmol) to the reaction tube equipped with a stir bar , put on the stopper and remove from the glove box, place it above a 30w, 460-470nm blue light at a distance of 3cm, and stir the reaction at a speed of 950r / min at room temperature until the reaction material imidate is completely detected by TLC thin layer chromatography disappear, stop stirring, and the reaction ends. Open the stopper and concentrate under reduced pressure using a rotary evaporator to remove volatile solvents, perform silica gel column chromatography, and concentrate under reduced pressure to obtain amino acid ester compounds with the structural formula (2a). White solid. Yield: 49.2 mg(90%), m.p. 105-107℃. 1 H NMR (400 MHz, Chloroform- d ) δ 7.54 – 7.47 (m, 2H),7.41 – 7.31 (m, 3H), 6.77 – 6.70 (m,...
Embodiment 2
[0060]
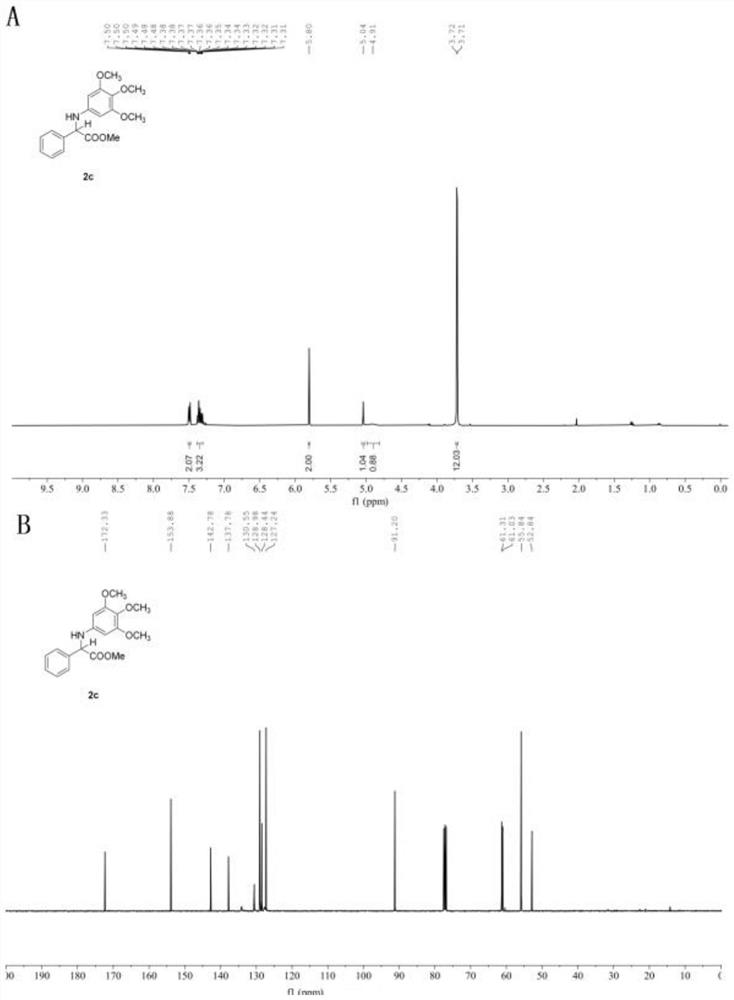
[0061] In the glove box, weigh the photosensitizer Ru(bpy) 3 Cl 2 (1.3mg, 1 mol%), the corresponding imidate 1c (0.2mmol), add acetonitrile (1.0mL), water (29mL, 1.6 mmol), phenylsilane (43.3mg, 0.4 mmol) to a stirring bar Put the stopper in the reaction tube, remove it from the glove box, place it above a 30w, 460-470 nm blue light at a distance of 3 cm, and stir the reaction at a speed of 900 r / min at room temperature until TLC thin-layer chromatography detects that the reaction raw material is sub- Amino acid ester disappears completely, stop stirring, and reaction finishes. Open the plug and use a rotary evaporator to remove volatile solvents under reduced pressure, perform silica gel column chromatography, and concentrate under reduced pressure to obtain amino acid ester compounds with the structural formula (2c). White solid. Yield: 63.3 mg (95%), m.p. 105-107℃. 1 H NMR (400 MHz, Chloroform- d ) δ 7.51 –7.47 (m, 2H), 7.39 – 7.30 (m, 3H), 5.80 (s, 2H), 5.0...
Embodiment 3
[0065]
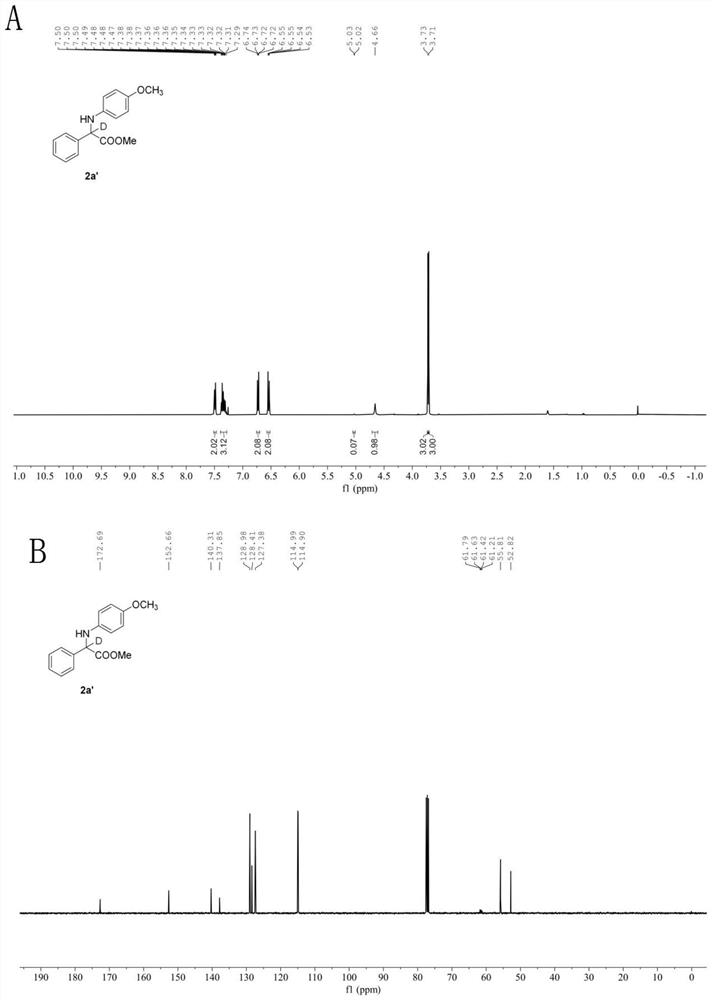
[0066] In the glove box, weigh the photosensitizer Ru(bpy) 3 Cl 2 (2.6mg, 2 mol%), the corresponding imidate 1f (0.2mmol), add acetonitrile (1.0mL), water (29mL, 1.6 mmol), phenylsilane (43.3mg, 0.4mmol) to a stirring bar Put the stopper in the reaction tube, remove it from the glove box, place it above a 30w, 460-470 nm blue light at a distance of 3 cm, and stir the reaction at a speed of 950 r / min at room temperature until TLC thin-layer chromatography detects that the reaction material is sub- Amino acid ester disappears completely, stop stirring, and reaction finishes. Open the stopper and use a rotary evaporator to remove volatile solvents under reduced pressure, perform neutral alumina chromatography, and concentrate under reduced pressure to obtain the deuterated amino acid ester compound with the structural formula (2f). White solid. Yield: 50.9 mg (86%), m.p. 115-117℃. 1 H NMR (400 MHz, Chloroform- d ) δ 7.53(d, J = 7.5 Hz, 2H), 7.36 (dt, J = 13.7,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com