Method for catalyzing indole silylation and proton transfer hydrogenation
A proton transfer, indole silane technology, applied in chemical instruments and methods, physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, etc. Due to the large amount of catalyst and other problems, it can achieve the effects of efficient and fast proton transfer hydrogenation, mild reaction conditions, and wide applicability of substrates.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
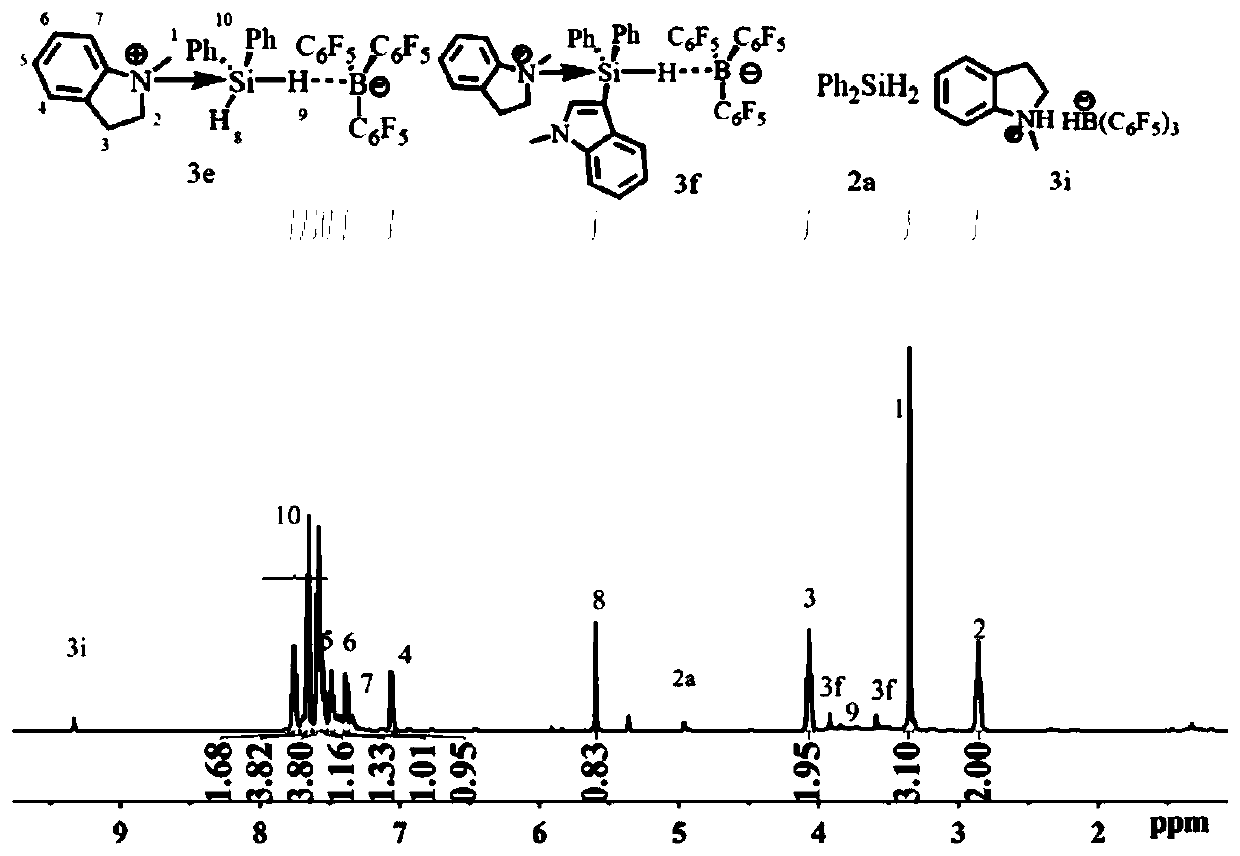
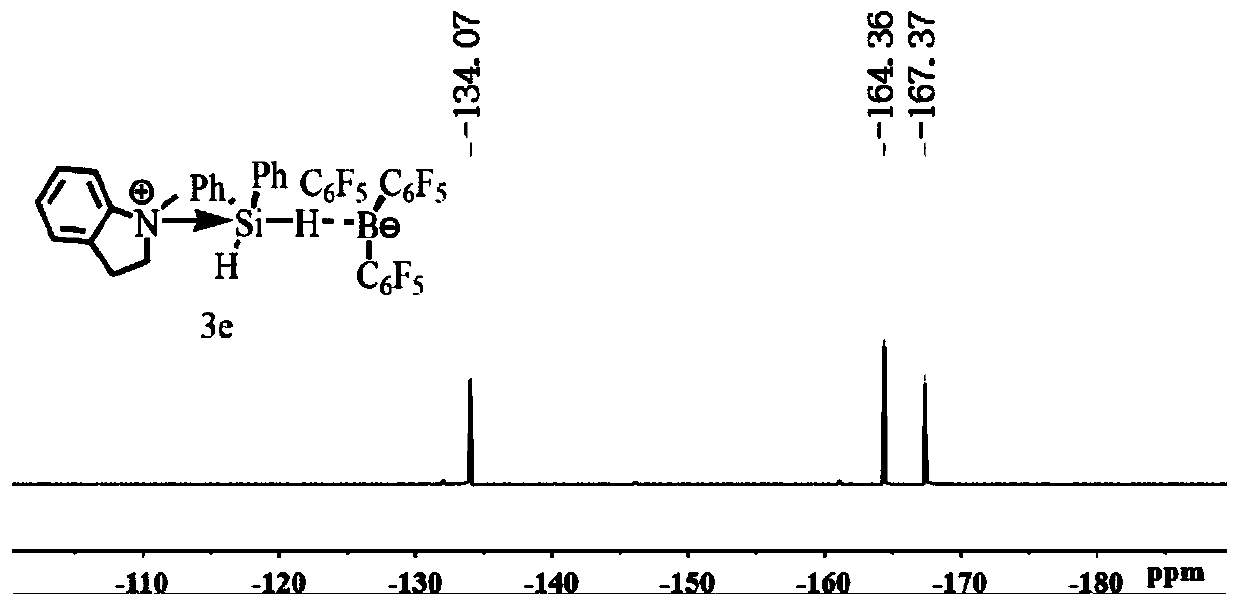
[0033] Embodiment 1 in situ nuclear magnetic generation C 9 h 11 N PhSiH 2 ·B(C 6 f 5 ) 3 (3e)
[0034]
[0035] Add B(C) to the J.Young-type NMR tube 6 f 5 ) 3 (12.8mg, 0.025mmol) and 0.3mL of CD 2 Cl 2 , add 0.1mL Ph 2 SiH 2 (4.6mg, 0.025mmol) and the CD of 0.2ml 1-methylindoline (3.3mg, 0.025mmol) 2 Cl 2 Solution, mix evenly, the solution is colorless after reacting for ten minutes, carry out NMR test, it can be clearly seen from the H NMR spectrum that the main product generated is C 9 h 11 N PhSiH 2 ·B(C 6 f 5 ) 3 (3e). ( 1 H / 19 See attached for F NMR chart figure 1 and 2 ) 1 H NMR (500MHz, CD 2 Cl 2 )δ7.78–7.71 (m,3H,H Ar ),7.71–7.67(m,2H,H Ar ),7.60–7.54(m,6H,H Ar ),7.42–7.33(m,4H,H Ar ),7.27(t,J=8.5Hz,1H,H Ar ),7.13(t,J=8.0Hz 1H,H Ar ),6.92(d,J=8.0Hz,1H,H Ar ),6.45(d,J=8.5Hz,1H,H Ar ), 4.92 (ddd, J=11.6, 9.4, 4.0Hz, 1H, NCH 2 ),3.92(s,3H,NCH 3 ),3.90–3.84(m,1H,NCH 2 ),3.66(br q,1H,BH),3.58(s,3H,NCH 3 ),3.21–3.12(m,1H,NCH 2 ), 2....
Embodiment 2
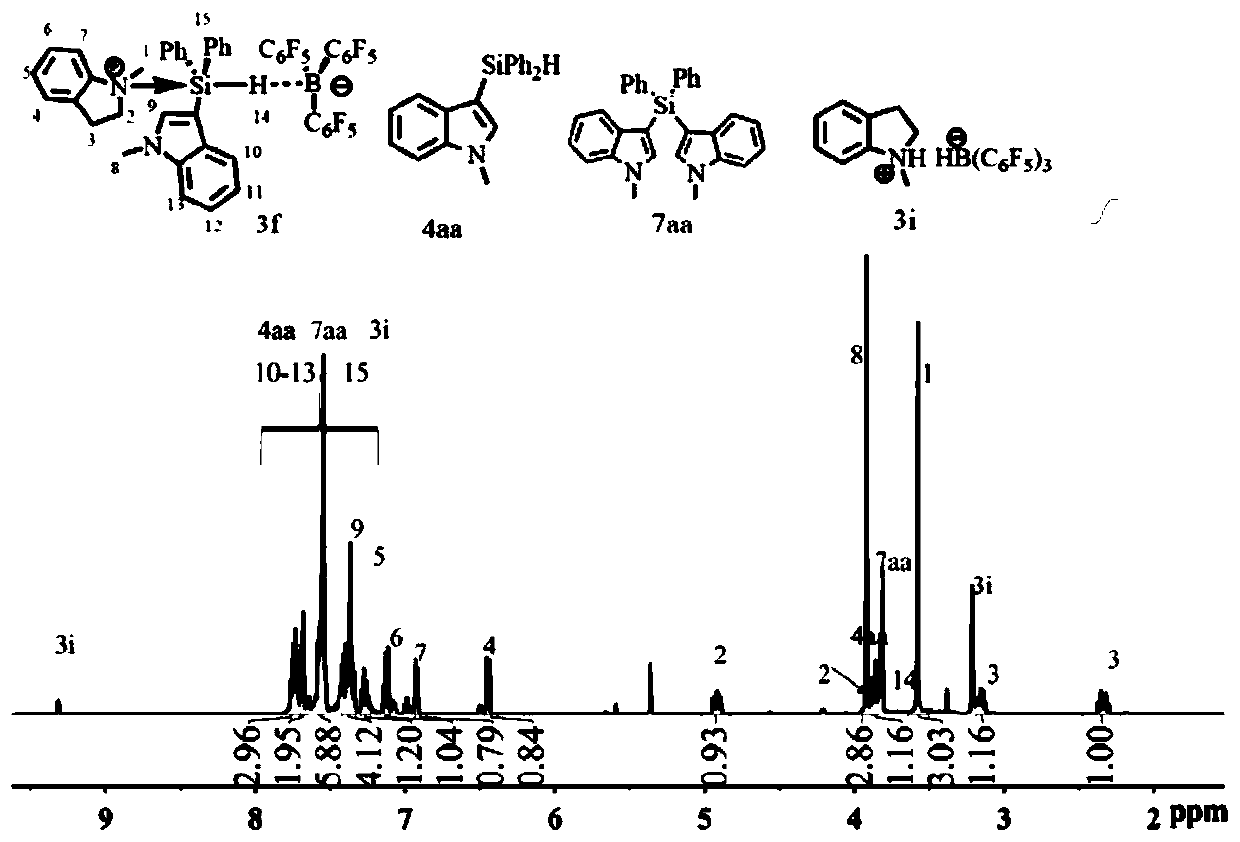
[0036] Embodiment 2 in situ nuclear magnetic generation C 9 h 11 N·C 21 h 18 NSiH·B(C 6 f 5 ) 3 (3f)
[0037]
[0038] Add B(C) to the J.Young-type NMR tube 6 f 5 ) 3 (12.8mg, 0.025mmol) and 0.3mL of CD 2 Cl 2 , add 0.1mL C into it with a syringe 21 h 18 CD of NSiH(4aa) (7.8mg, 0.025mmol) and 0.2ml 1-methylindoline (3.3mg, 0.025mmol) 2 Cl 2 Solution, mixed evenly, the solution was colorless after ten minutes of reaction, nuclear magnetic test was carried out, it can be clearly seen from the hydrogen nuclear magnetic spectrum that the main product generated is C 9 h 11 N·C 21 h 18 NSiH·B(C 6 f 5 ) 3 (3f). ( 1 H / 19 See attached for F NMR chart image 3 and 4 ) 1 H NMR (500MHz, CD 2 Cl 2 )δ7.78–7.71 (m,3H,H Ar ),7.71–7.67(m,2H,H Ar ),7.60–7.54(m,6H,H Ar ),7.42–7.33(m,4H,H Ar ),7.27(t,J=8.5Hz,1H,H Ar ),7.13(t,J=8.0Hz1H,H Ar ),6.92(d,J=8.0Hz,1H,H Ar ),6.45(d,J=8.5Hz,1H,H Ar ), 4.92 (ddd, J=11.6, 9.4, 4.0Hz, 1H, NCH 2 ),3.92(s,3H,NCH 3 ),3.9...
Embodiment 3
[0040] Preparation of 3-(diphenylsilyl)-1-methylindole and 1-methylindoline under the conditions of embodiment 3 without solvent
[0041]
[0042] In the glove box, weigh 1-methylindole (13.1g 0.1mol), B(C 6 f 5 ) 3 (0.01mmol, 5.6mg) was fully stirred in a 30mL reaction flask, when B(C 6 f 5 ) 3 After complete dissolution, add Ph 2 SiH 2 (9.2g0.05mol), stirring at room temperature for 24h, taking 0.2mL reaction solution with a pipette gun and dissolving it in deuterated benzene, calculating the conversion rate of the reaction by the integrated area of the raw material and the product nitrogen methyl group on the proton nuclear magnetic spectrum to be 96.9%, The yields of 3-(diphenylsilyl)-1-methylindole and 1-methylindoline were 46.1% and 45.2%, respectively. Post-processing method: Pour the reaction mixture into 300 mL of hexane, stir for 30 minutes, filter to obtain a white solid, wash with (3×30 mL) hexane and dry it, and obtain a white solid 3-(diphenylsilyl)- ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com