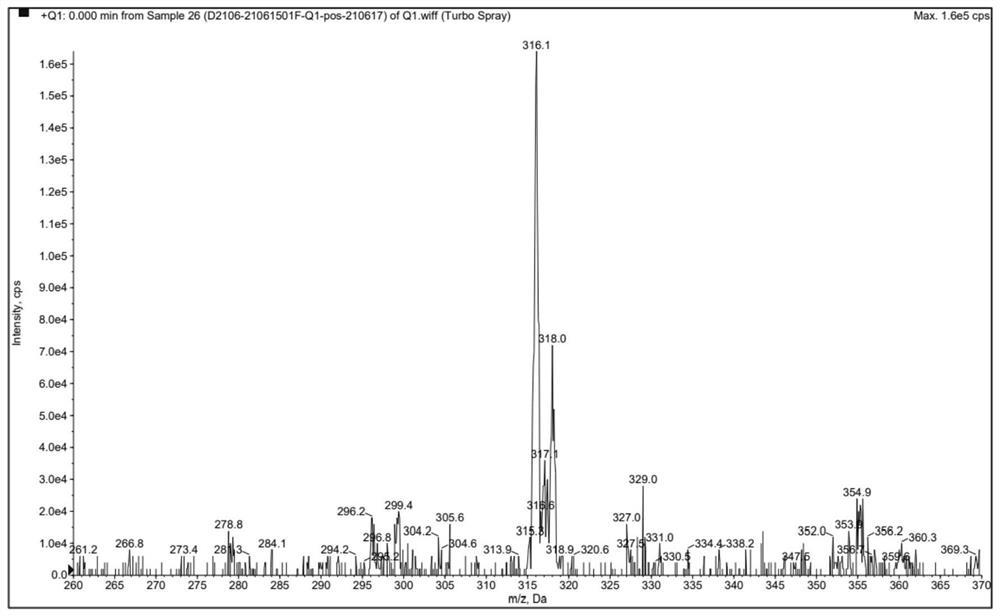
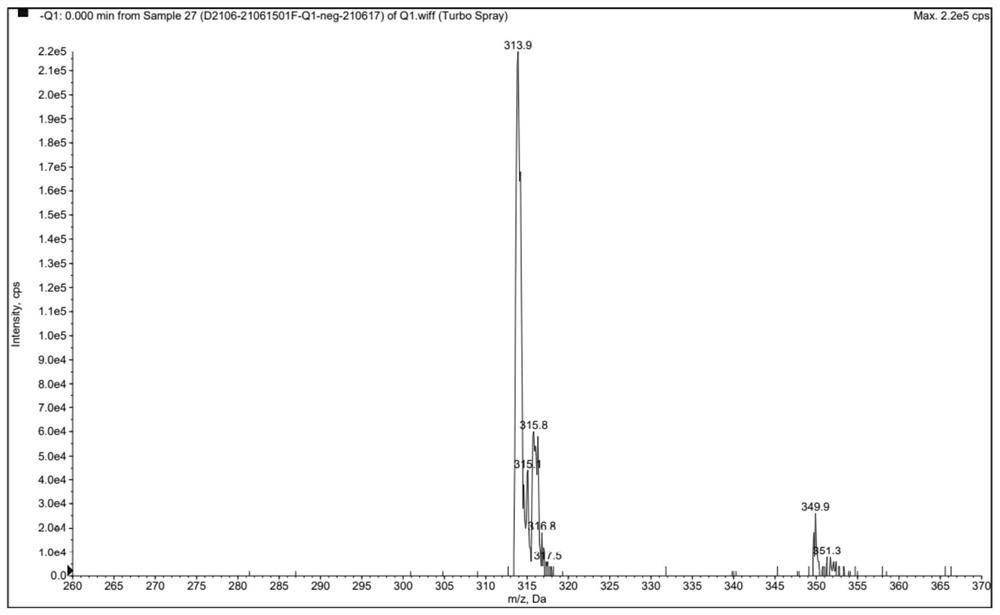
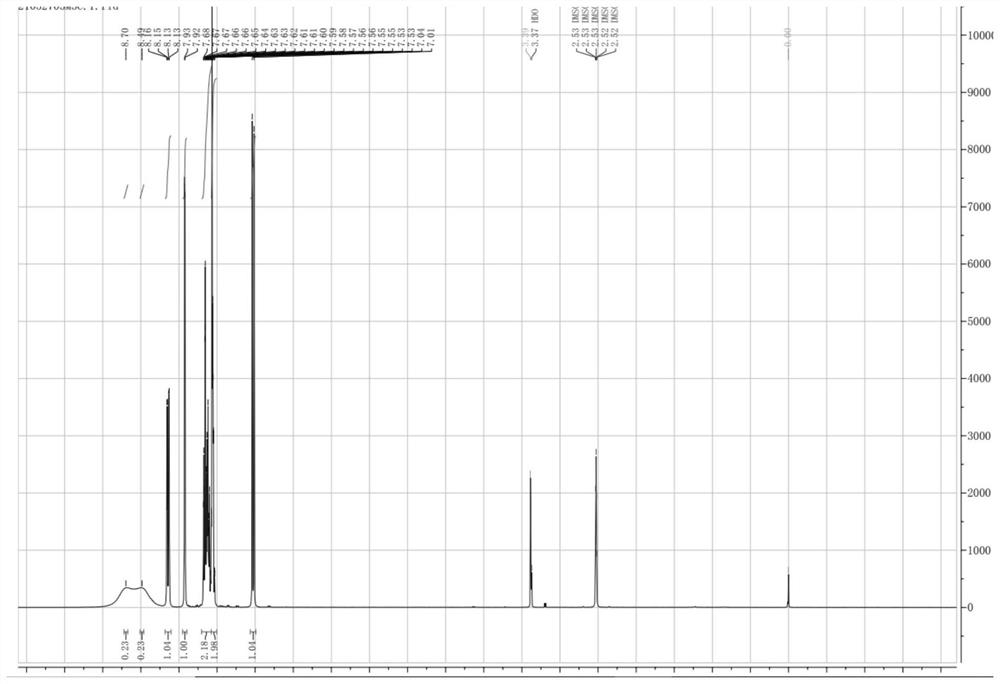
Preparation method of clonazepam related substance B
A related substance, clonazepam technology, applied in the field of preparation of clonazepam related substance B, can solve the problems such as less amount of impurity A, complicated separation, easy overreaction, etc. Simple steps and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The preparation method of clonazepam related substance B comprises the following steps:
[0045] Add chloride (10.00g, 28.32mmol) to the reaction flask, add 110ml of methanol and stir to dissolve, the system is off-white turbid, add ammonia water (30ml, 212.40mmol) dropwise at 25°C, after the addition is complete, the system becomes light yellow turbid system, continue stirring at room temperature for 1 h.
[0046] TLC showed that the reaction was complete, 70ml of water was added dropwise to the reaction system, filtered, and dried at 40°C to obtain a light yellow powder weighing 7.47g with a yield of 65.3%. The HPLC test results showed that the purity was 98.55%.
Embodiment 2
[0048] The preparation method of clonazepam related substance B comprises the following steps:
[0049] Add chloride (10.00g, 28.32mmol) to the reaction bottle, add methanol 110ml and stir to dissolve, the system is off-white turbidity, 7M ammonia methanol solution (20ml, 141.60mmol) is added dropwise at 25°C, after the addition is complete, the system is pale Yellow turbidity, continue to stir at room temperature for 1h, the system dissolves and becomes clear, and it is a light yellow solution.
[0050] TLC showed that the reaction was complete, methanol was concentrated to obtain a light yellow solid, and air-dried to obtain a light yellow powder weighing 10.06 g with a yield of 88.0%. The HPLC test results showed that the purity was 99.67%.
Embodiment 3
[0052] The preparation method of clonazepam related substance B comprises the following steps:
[0053] Add chloride (10.00g, 28.32mmol) to the reaction flask, add methanol 110ml and stir to dissolve, the system is off-white turbidity, 7M ammonia methanol solution (20ml, 141.60mmol) is added dropwise at 20°C, after the addition is complete, the system is pale Yellow turbidity, continue to stir at room temperature for 1h, the system dissolves and becomes clear, and it is a light yellow solution.
[0054] TLC showed that the reaction was complete, 90ml of water was added dropwise to the reaction system, filtered, and dried at 40°C to obtain a light yellow powder weighing 7.50g with a yield of 66.0%. The HPLC test results showed that the purity was 99.28%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com