Orthoester miscible substance pharmaceutic adjuvant, preparation method and local sustained-release drug delivery preparation containing orthoester miscible substance pharmaceutic adjuvant
A technology of pharmaceutical excipients and orthoesters, which is used in pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc. To solve problems such as sexuality, to achieve the effect of simple preparation method, improved therapeutic index and compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
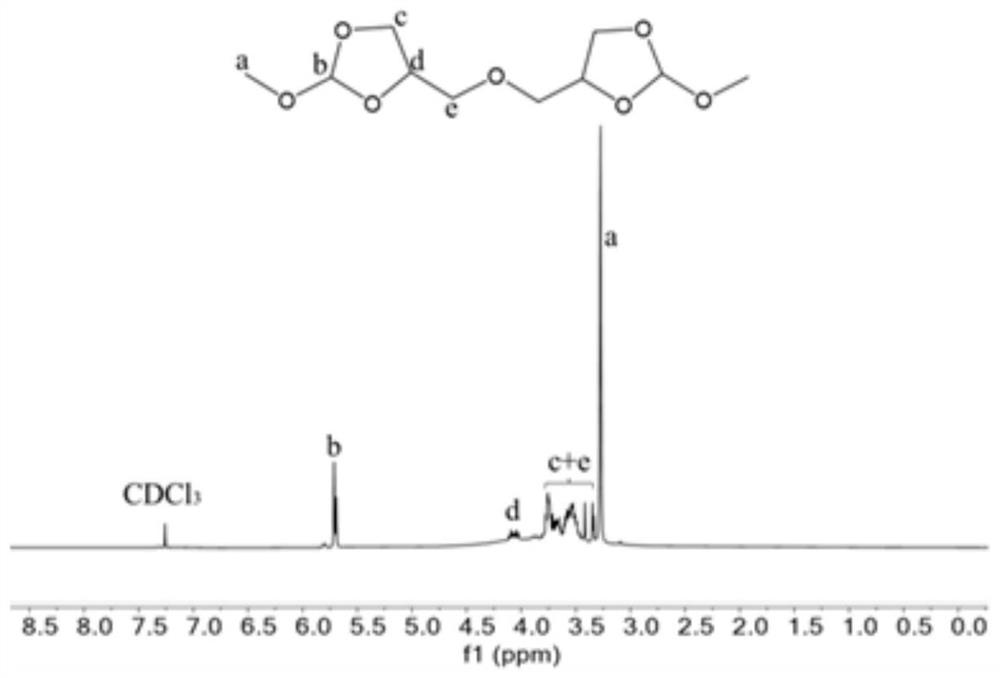
[0074] Synthesis of orthoester compound 4,4'-(oxybis(methylene))bis(2-methoxy-1,3-dioxolane)(OE-1)
[0075] Under nitrogen protection, diglycerol (16.6g, 0.1mol), trimethyl orthoformate (31.84g, 0.3mol) and p-toluenesulfonic acid (344.4mg, 0.002mol) were added to the reaction flask, and acetonitrile (150mL ) was dissolved and reacted overnight at room temperature. After the crude product was distilled off under reduced pressure to remove acetonitrile, ethyl acetate was added to dissolve it, extracted with saturated sodium carbonate solution, dried over anhydrous magnesium sulfate, ethyl acetate and excess trimethyl orthoformate were distilled off under reduced pressure to obtain a colorless oily product, The rate is 83%, 1 H NMR as figure 1 shown.
Embodiment 2
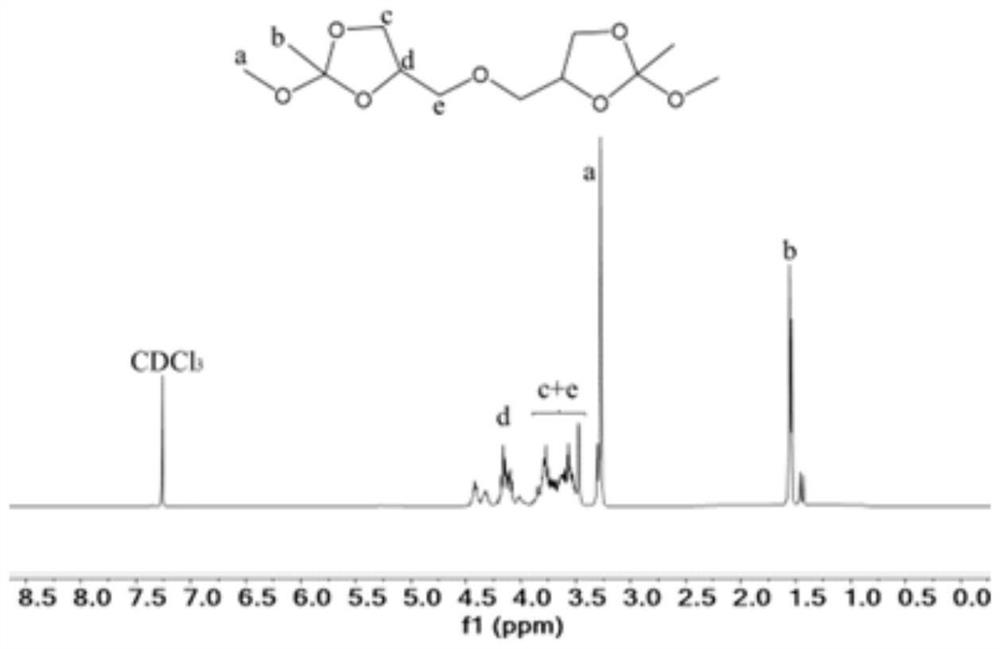
[0077] Synthesis of ortho ester compound 4,4'-(oxybis(methylene))bis(2-methoxy-4-methyl-1,3-dioxolane)(OE-2)
[0078] Under nitrogen protection, diglycerol (16.6g, 0.1mol), trimethyl orthoacetate (36.05g, 0.3mol) and p-toluenesulfonic acid (344.4mg, 0.002mol) were added to the reaction flask, and acetonitrile (150mL ) was dissolved and reacted overnight at room temperature. After the crude product was distilled off under reduced pressure to remove acetonitrile, ethyl acetate was added to dissolve it, extracted with saturated sodium carbonate solution, dried over anhydrous magnesium sulfate, ethyl acetate and excess trimethyl orthoformate were distilled off under reduced pressure to obtain a colorless oily product, The rate is 78%, 1 H NMR as figure 2 shown.
Embodiment 3
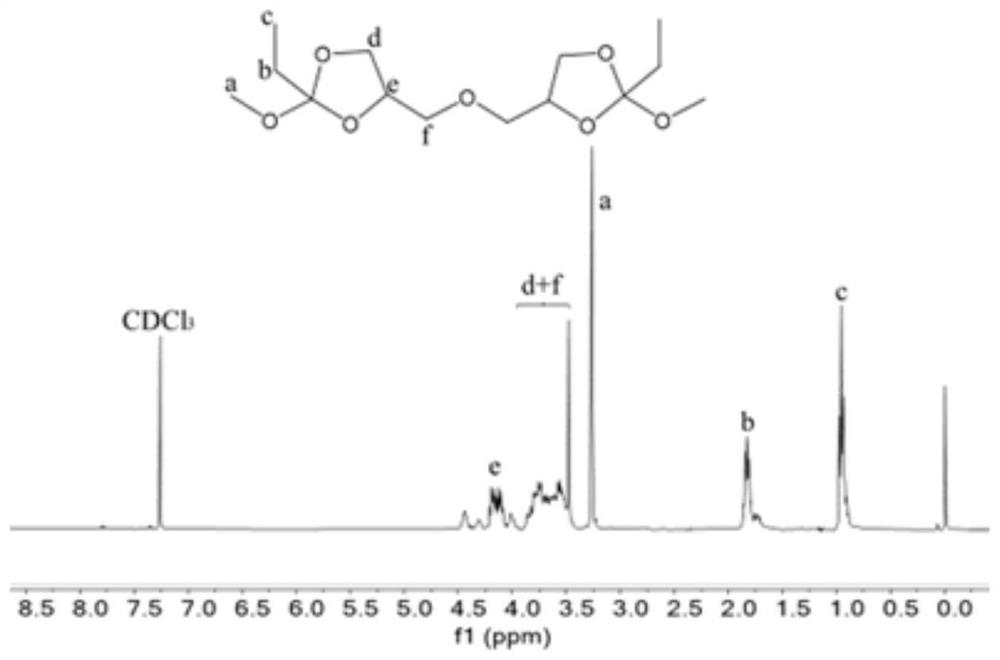
[0080] Synthesis of ortho ester compound 4,4'-(oxybis(methylene))bis(4-ethyl-2-methoxy-1,3-dioxolane)(OE-3)
[0081] Under nitrogen protection, diglycerol (16.6g, 0.1mol), trimethyl orthopropionate (40.25g, 0.3mol) and p-toluenesulfonic acid (344.4mg, 0.002mol) were added to the reaction flask, and dichloro Methane (150 mL) was dissolved and reacted overnight at room temperature. After the crude product was distilled off under reduced pressure to remove acetonitrile, ethyl acetate was added to dissolve it, extracted with saturated sodium carbonate solution, dried over anhydrous magnesium sulfate, ethyl acetate and excess trimethyl orthoformate were distilled off under reduced pressure to obtain a colorless oily product, The rate is 81%, 1 H NMR as image 3 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com