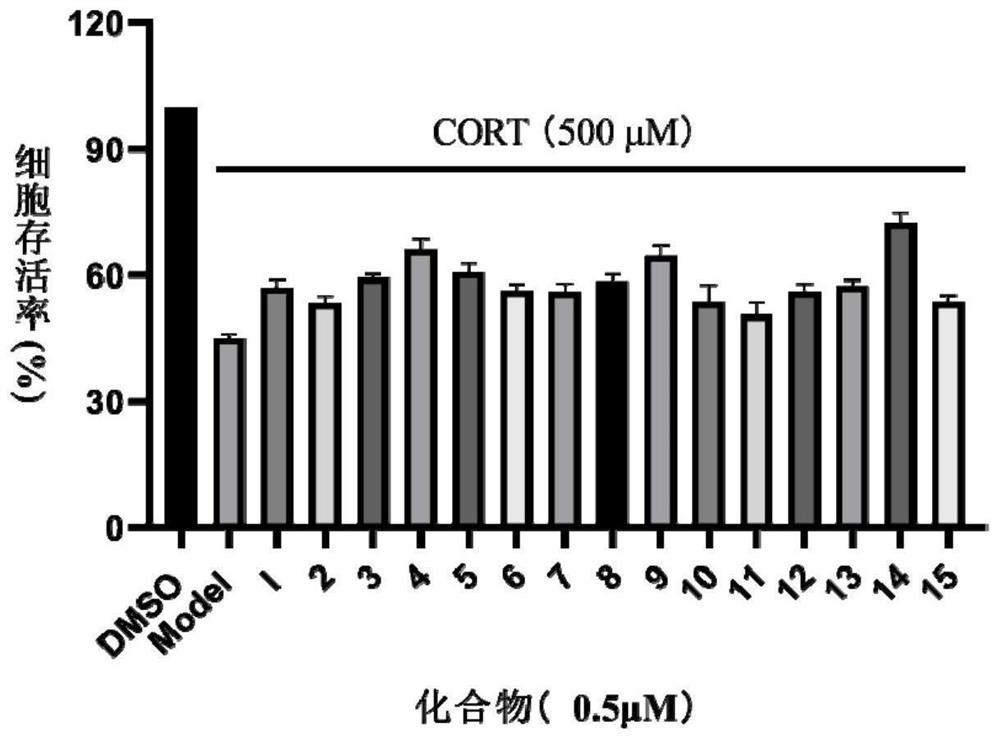
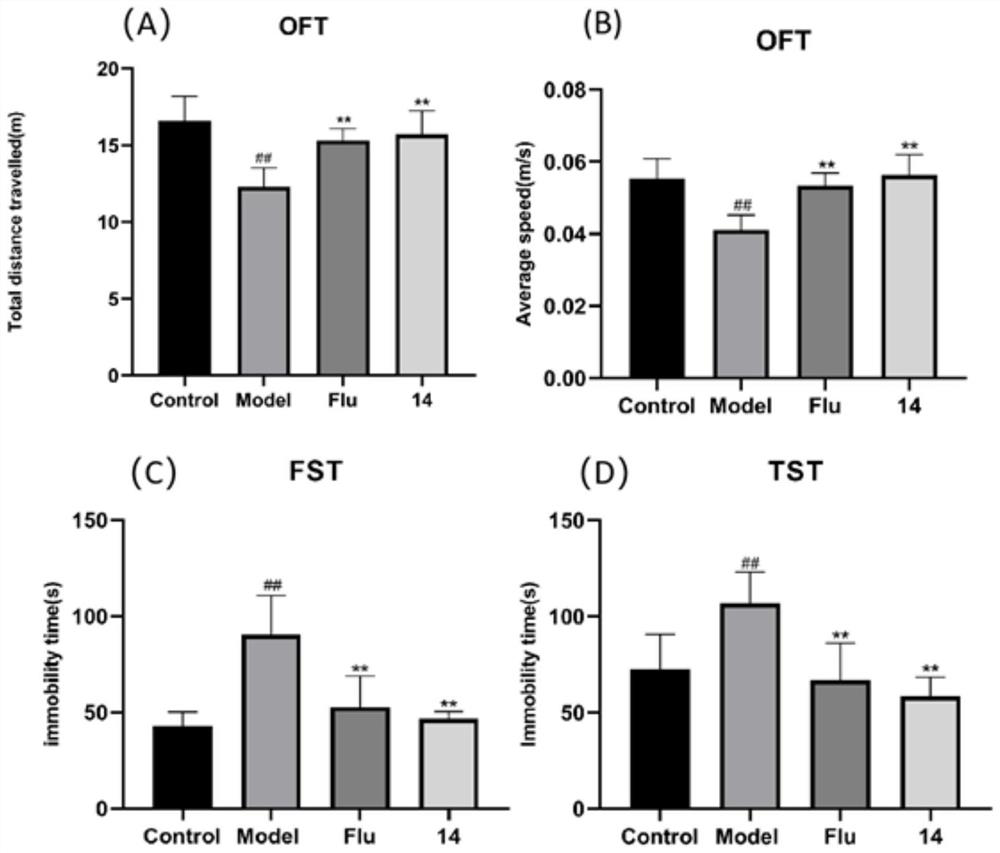
Oxidized isoaporphine alkaloid derivative as well as preparation method and anti-depression application thereof
An alkaloid derivative, the technology of isoapofil, which is applied in the field of medicinal chemistry, can solve the problems of poor patient compliance and no expected treatment response, and achieve the effects of improving depression, good protective activity, and low toxicity of reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: 5-[[1-(2-chlorophenyl) piperazine] ethoxy] -1- azabenzanthrone (compound I 1 )Synthesis
[0043] The reaction formula is as follows:
[0044]
[0045] Dissolve 5-hydroxyl-1-azabenzanthrone (247mg, 1mmol, 1eq) in 10ml acetone, add K 2 CO 3 (207mg, 1.5mmol, 1.5eq) and 1,2-dibromoethane (335mg, 1.8mmol, 1.8eq), stirred overnight at 50°C, stopped the reaction, dried under reduced pressure to remove the solvent, and subjected to silica gel column chromatography (eluent PE:EA=8:1V:V) purification to obtain intermediate 5-(2-bromoethoxy)-1-azabenzanthrone; then 5-(2-bromoethoxy)-1- Azabenzanthrone (141mg, 0.4mmol, 1eq) was added to the reaction flask, dissolved in 10ml of acetonitrile, and K 2 CO 3 (166mg, 1.2mmol, 3eq), KI (33mg, 0.2mmol, 0.5eq) and 1-(2-chlorophenyl)piperazine (157mg, 0.8mmol, 2eq), stirred overnight at 60°C, stopped the reaction, and reduced pressure The solvent was removed by drying, and purified by silica gel column chromatography (...
Embodiment 2
[0047] 1 H NMR (500MHz, CDCl 3)δ8.88(d, J=7.8Hz, 1H), 8.68(d, J=5.4Hz, 1H), 8.40(d, J=7.7Hz, 1H), 8.29(d, J=1.9Hz, 1H) ,7.80(t,J=7.3Hz,1H),7.63(dd,J=13.3,6.4Hz,2H),7.43(d,J=1.7Hz,1H),7.36(d,J=7.8Hz,1H) ,7.22(t,J=7.2Hz,1H),7.06(d,J=7.8Hz,1H),6.97(t,J=7.2Hz,1H),4.38(t,J=4.7Hz,2H),3.14 (s, 4H), 3.03 (t, J=4.5Hz, 2H), 2.86 (s, 4H). Example 2: 5-[[1-(2-methoxyphenyl)piperazine]ethoxy] -1-azabenzoanthrone (compound I 2 )Synthesis
[0048] The reaction formula is as follows:
[0049]
[0050] Reference compound I 1 The preparation method, replace compound 1-(2-chlorophenyl) piperazine with 1-(2-methoxyphenyl) piperazine, other conditions are unchanged, through silica gel column chromatography (eluent is DCM:MeOH= 30:1V:V) obtain target compound I 2 , yellow solid, yield 61%.
[0051] ESI-MS: 466.2.[M+H] + .
[0052] 1 H NMR (500MHz, CDCl 3 )δ8.88(d, J=7.7Hz, 1H), 8.68(d, J=5.6Hz, 1H), 8.40(d, J=7.2Hz, 1H), 8.29(d, J=2.2Hz, 1H) ,7.80(t,J=7.0Hz,1H),7.63(dd,J=12.8,6.3Hz...
Embodiment 3
[0053] Embodiment 3: 5-[[(2,3-dichlorophenyl) piperazine] ethoxy] -1- azabenzanthrone (compound I 3 )Synthesis
[0054] The reaction formula is as follows:
[0055]
[0056] Reference compound I 1 The preparation method, replace compound 1-(2-chlorophenyl) piperazine with 1-(2,3-dichlorophenyl) piperazine, other conditions are constant, through silica gel column chromatography (eluent is DCM: MeOH=25:1V:V) to obtain target compound I 3 , yellow solid, yield 58%.
[0057] ESI-MS: 504.1[M+H] + .
[0058] 1 H NMR (500MHz, CDCl 3 )δ8.88(d, J=7.7Hz, 1H), 8.68(d, J=5.5Hz, 1H), 8.40(d, J=7.6Hz, 1H), 8.29(s, 1H), 7.81(t, J=7.2Hz, 1H), 7.63(dd, J=13.3, 6.4Hz, 2H), 7.43(s, 1H), 7.15(d, J=7.2Hz, 2H), 6.97(d, J=5.3Hz, 1H), 4.38(s, 2H), 3.13(s, 4H), 3.03(s, 2H), 2.87(s, 4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com