Synthetic method of hexabenzoquinone dimer
A technology of benzocoronet dimer and synthesis method, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as insufficient stability of intermediates, low total yield of routes, harsh conditions, etc. , to achieve the effect of regular molecular arrangement, widening range and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
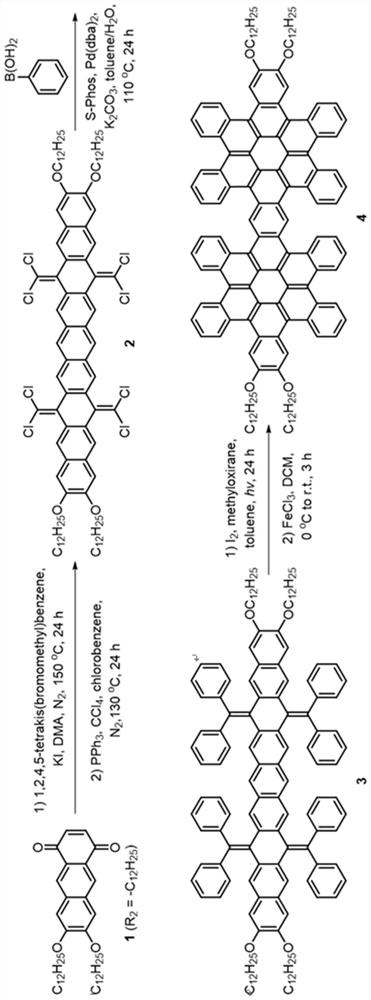
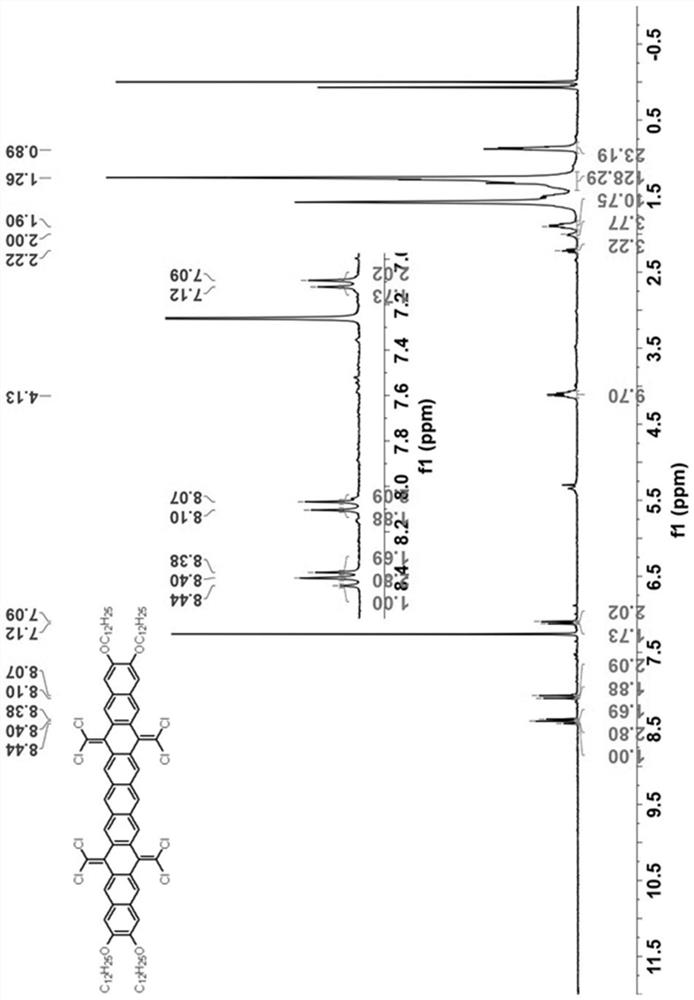
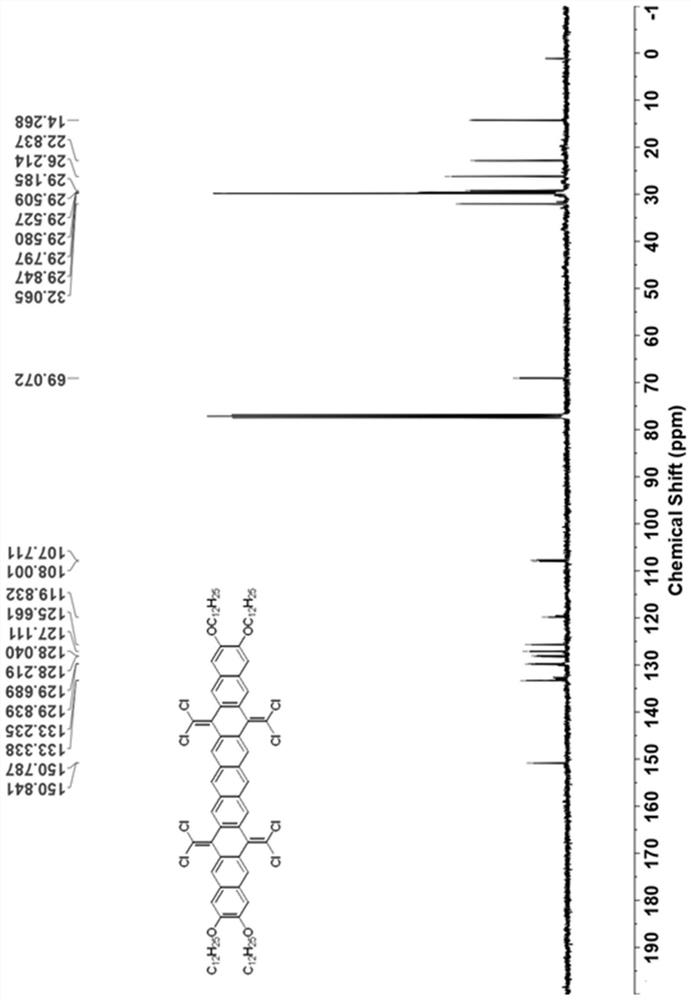
[0049] see figure 1 As shown in the technical process, this embodiment provides a synthetic method of hexabenzocoronene dimer, using the following steps:
[0050] (1) 7,8-bis(dodecyloxy)anthracene-1,4-dione (compound 1) was synthesized according to literature. (References: Org. Lett. 2009, 11(11), 2225-2228.)
[0051] (2) Under nitrogen atmosphere, add 1,2 , 4,5-Tetrakis(bromomethyl)benzene (2.25g, 5mmol, 1.0eq) and potassium iodide (9.96g, 60mmol, 12.0eq). The brown suspension was stirred at 150° C. under nitrogen for 24 hours, then 100 mL of methanol was added at room temperature. The resulting brown solid was filtered and dried. The insoluble solid was used in the next step without further purification.
[0052] Under nitrogen atmosphere, the above solid was dissolved in 100 mL of chlorobenzene, then triphenylphosphine (15.74 g, 60 mmol, 12.0 eq) and carbon tetrachloride (9.64 mL, 100 mmol, 20.0 eq) were added. The solution was refluxed for 24 hours under the protecti...
Embodiment 2
[0064] Compared with Example 1, most of them are the same, except that in this example, 7,8-bis(dodecyloxy)anthracene-1,4-dione, 1,2,4,5-tetra( The molar weights of bromomethyl)benzene, potassium iodide, triphenylphosphine and carbon tetrachloride were adjusted to 2eq, 10eq, 10eq, 18eq respectively.
Embodiment 3
[0066] Compared with Example 1, most of them are the same, except that in this example, 7,8-bis(dodecyloxy)anthracene-1,4-dione, 1,2,4,5-tetra( The molar weights of bromomethyl)benzene, potassium iodide, triphenylphosphine and carbon tetrachloride were adjusted to 2.5eq, 14eq, 14eq, 22eq respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com