Improved inhalable agglomerates
A technology of aggregates and drugs, applied in the field of medicine, can solve the problems of unfavorable drug clinical applications, side effects, and decreased efficiency of drug delivery to the lungs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0119] The preparation of embodiment 1 micronization active agent particle
[0120] The active agent is micronized using the MCONE pulverizer of Italian DEC company, and parameters such as crushing pressure, feeding pressure and feeding speed are limited according to the task requirements, so as to obtain the active agent particles with the target particle size. The particle size was measured using a SYMPATEC particle size analyzer with a 4 bar dispersion pressure. The particle size results are shown in Table 1 and Table 2.
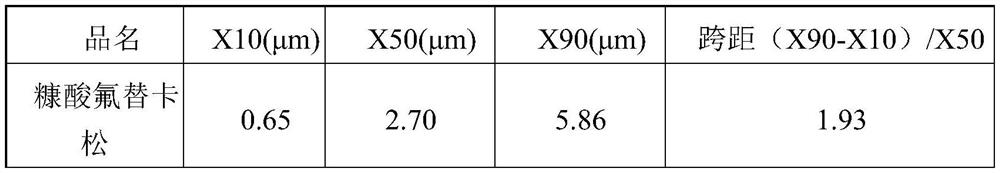
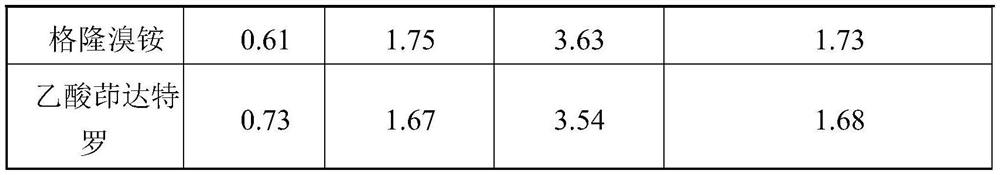
[0121] Table 1 API particle size distribution
[0122]
[0123]
[0124] Table 2 API particle size distribution
[0125] X10(μm) X50(μm) X90(μm) fluticasone furoate 0.52 1.98 4.43 Glycopyrrolate 0.61 1.83 3.71 indacaterol acetate 0.68 1.93 3.68
Embodiment 2 3
[0126] The preparation of embodiment 2 three-component inhalation dry powder A
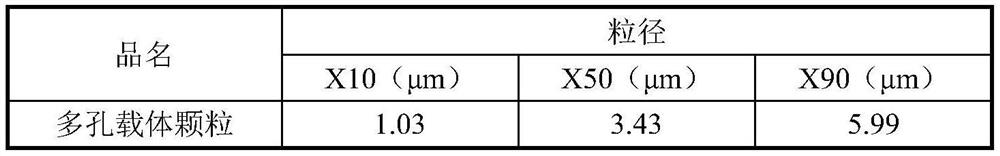
[0127] Disperse 40 g of distearoylphosphatidylcholine (DSPC) and 3.72 g of calcium chloride dihydrate in hot deionized water (T=70° C.), using an ULTRA-TURRAX TM high-shear mixer (T-25 model )8000rpm for 3 minutes. Subsequently, an appropriate amount of PFOB was added dropwise to the resulting DSPC / CaCl while shearing and mixing. 2 In the dispersion, the resulting DSPC / CaCl 2 The / PFOB dispersion was subjected to high-pressure homogenization for three rounds to obtain a blank carrier emulsion. The resulting emulsion is spray-dried on a BüCHI B-290 micro-spray dryer, spray-drying parameters: inlet temperature = 120°C, peristaltic pump speed: 5-7rpm (~ 2mL / min), fan frequency: 100%, atomizer flow Velocity = 60 cm (rotameter), to obtain porous carrier particles, the particle size of the carrier particles is shown in Table 3.
[0128] Table 3 particle size distribution of porous carrier particles ...
Embodiment 3 3
[0132] The preparation of embodiment 3 three-component inhalation dry powder B
[0133] Porous carrier particles were prepared by a method similar to that of Example 2, except that the target amount of fluticasone furoate was dispersed in hot deionized water together with distearoylphosphatidylcholine (DSPC) and calcium chloride dihydrate. The resulting porous carrier particles comprise fluticasone furoate as a core embedded in a porous phospholipid interface layer.
[0134] Weigh the indacaterol acetate, glycopyrronium bromide and drug-containing porous carrier in Table 1 that have been micronized, spray-dry them on a BüCHI B-290 micro-spray dryer after high-shearing in perfluorooctylbromide antisolvent, and enter Temperature=140°C, peristaltic pump speed: 5-7rpm (~2mL / min), fan frequency: 100%, atomizer flow rate=46cm (rotameter), and inhalation dry powder B was obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com