Synergistic extraction system for co-extraction of nickel and cobalt and co-extraction method of co-extraction system
A nickel-cobalt co-extraction and system technology, applied in the field of metal ion extraction system, can solve the problems of high-efficiency separation of difficult-to-interference metal ions, high-efficiency stripping of nickel-cobalt, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] The extraction and stripping in this embodiment were carried out at a temperature of 25°C.
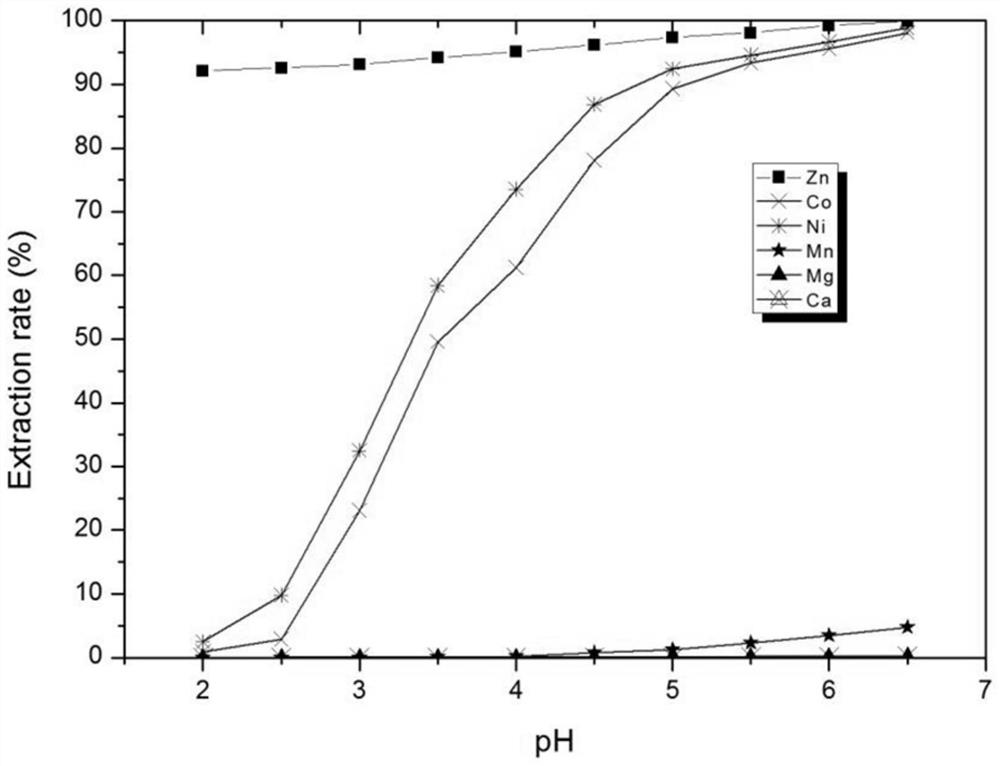
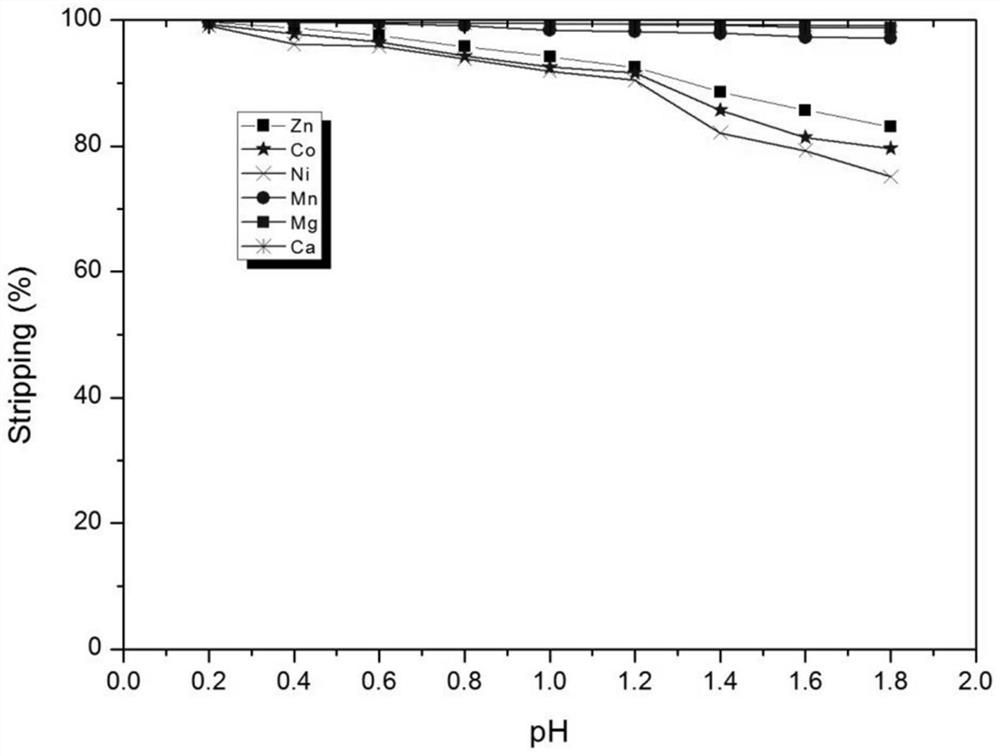
[0052] Composition of water phase simulated feed liquid (to-be-treated liquid): cobalt: 1.05g / l, nickel: 2.93g / L, zinc: 0.33, g / L, manganese: 3.18g / L, magnesium: 12.02g / L, calcium: 1.69g / L, the pH value of the feed liquid is 2.51.
[0053] The initial organic phase composition: with hydrogenated kerosene as diluent, the concentration of neodecanoic acid in the organic phase is 0.5mol / L, the concentration of 4-hydroxy-6-ethyl-decane-5-oxime is 0.35mol / L, N , the concentration of N-didecyldecylamine is 0.4mol / L.
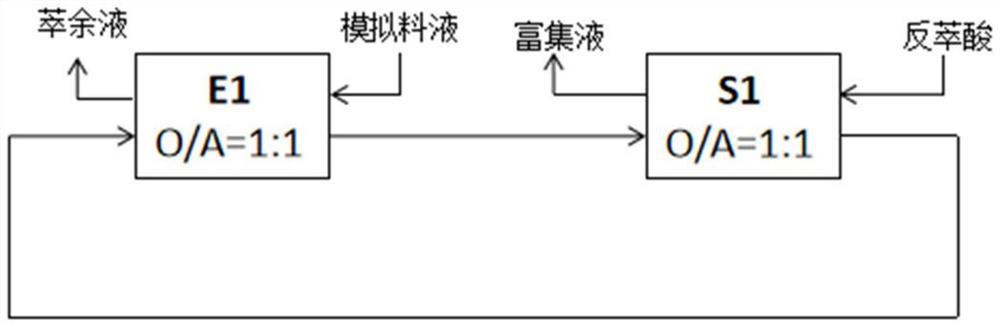
[0054] (1) Zinc extraction and stripping
[0055] Mix the above initial organic phase with the simulated feed liquid (liquid to be treated) at O / A=1:1 (10:1.0-1.0:10 can be selected according to the actual situation) at room temperature at 25°C for 5 minutes (it can be adjusted according to the actual situation Select 0.5-10 minutes), control the equilibrium pH value ...
Embodiment 2
[0066] The extraction and stripping in this embodiment were carried out at a temperature of 30°C. The composition of the simulated water phase liquid is consistent with that of Example 1.
[0067] The initial organic phase composition: with hydrogenated kerosene as diluent, the content of naphthenic acid (hexahydrobenzoic acid) in the initial organic phase is 0.45mol / L, 5-diethyl-7-hydroxyl-6-dodecyl oxime The content is 0.32mol / L, and the content of trioctylamine is 0.35mol / L.
[0068] (1) Zinc extraction and stripping
[0069] The experimental procedure was the same as in Example 1, and the results shown in Table 3 were obtained.
[0070] Table 3: The effect of the co-extraction system "naphthenic acid + 5-hydroxy-7-ethyl-undecane-6-oxime + trioctylamine" on the separation of zinc and nickel and cobalt
[0071] metal element cobalt nickel zinc manganese magnesium calcium Extraction rate (%) 0.92 3. 92.86 0.0 0.0 0.0 Stripping rate (%)...
Embodiment 3
[0080] The extraction and stripping in this example were carried out at a temperature of 10°C. The composition of the simulated water phase liquid is consistent with that of Example 1.
[0081] Initial organic phase composition: with hydrogenated kerosene as diluent, the content of naphthenic acid (hexahydrobenzoic acid) in the initial organic phase is 0.52mol / L, and the content of 5-hydroxyl-7-ethyl-undecane-6-oxime is 0.37mol / L, the content of methyl tributyl ammonium bromide is 0.50mol / L.
[0082] (1) Zinc extraction and stripping
[0083] The experimental procedure was consistent with that of Example 1, and the results shown in Table 5 were obtained.
[0084] Table 5. The effect of the co-extraction system "naphthenic acid + 5-hydroxy-7-ethyl-undecane-6-oxime + methyltributylammonium bromide" on the separation of zinc and nickel-cobalt
[0085] metal element cobalt nickel zinc manganese magnesium calcium Extraction rate (%) 1.02 3.59 91.25 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com