Novel carbonic anhydrase IX targeted photosensitizer and application thereof in field of medicine
A kind of technology of pheophorbide and chlorophyllin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
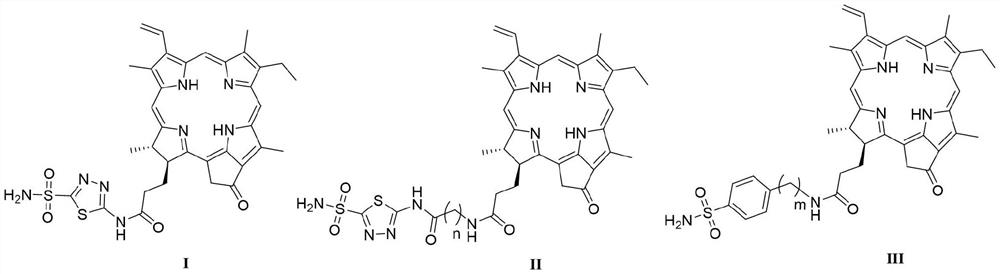
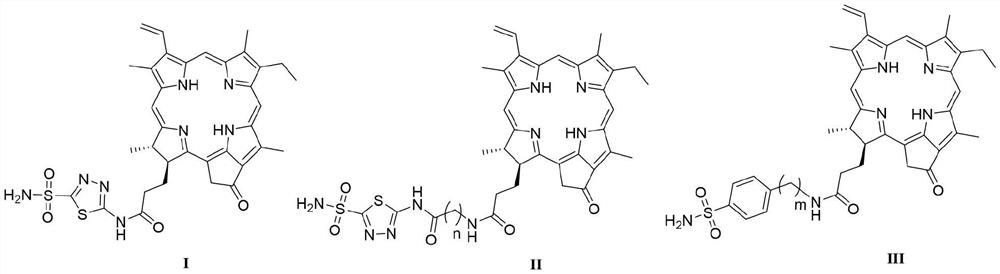
[0036] Preparation of N-(5-sulfamoyl-1,3,4-thiadiazol-2-yl) pyropheophytin (I):
[0037]
[0038]Add pyropheophorbide-a (107 mg, 200 μmol) and 40 mL of anhydrous acetonitrile into a round bottom flask, then add 1,3,4-thiadiazole-2-sulfonamide (52 mg, 240 μmol), and then add three Ethylamine (0.1 mL, 800 μmol), stirred at room temperature for 30 min. Finally, HATU (56 mg, 240 μmol) was added, and the reaction was stirred at room temperature for 16 h. After the completion of the reaction was monitored by TLC, 100-200 mL of saturated sodium chloride solution was added, and then 2M HCl solution was added to adjust the pH to acidity. Then it was extracted with ethyl acetate (100 mL×3) and washed with water. The organic phase was collected and washed with anhydrous Na 2 SO 4 Dry, filter, and evaporate the solvent under reduced pressure. The obtained residue was separated and purified by column chromatography to obtain compound I (74 mg, 53%) as a green solid powder. 1 H NMR ...
Embodiment 2
[0040] N-[2-((5-sulfamoyl-1,3,4-thiadiazol-2-yl)amino)-2-oxoethyl]pyropheophytin (II 1 ) preparation:
[0041]
[0042] Compound II was prepared by the synthetic method of compound I 1 . 1 H NMR (400MHz, DMSO-d 6 )δ: 13.10(s,1H), 9.74(s,1H), 9.46(s,1H), 8.92(s,1H), 8.38(s,1H), 8.30(s,2H), 8.29–8.17(m ,1H),6.40(d,J=17.8Hz,1H),6.22(d,J=11.6Hz,1H),5.25(d,J=20.2Hz,1H),5.13(d,J=20.1Hz,1H ),4.61(d, J=7.6Hz,1H),4.35(s,1H),4.06(s,2H),3.80–3.66(m,2H),3.63(s,3H),3.45(s,3H) ,3.23(s, 3H),2.67(s,1H),2.33(s,1H),2.20(s,2H),1.80(d,J=7.3Hz,3H),1.64(d,J=7.5Hz, 3H),0.28(s,1H),-1.94(s,1H). 13 C NMR (151MHz, DMSO-d 6 )δ:195.28,172.76,172.13,170.01,164.03, 161.43,153.69,149.61,147.81,144.24,140.57,136.88,135.49,134.96,134.66,131.50,128.91, 127.50,122.52,105.87,103.63,96.11,93.61,51.23 , 49.38, 47.55, 42.50, 32.29, 30.21, 22.90, 18.38, 17.33, 11.93, 11.52, 10.59. HRMS (MALDI-TOF) m / z: calcd for C 37 h 40 N 9 o 5 S 2 [M] + 754.25883, found 754.25598.
Embodiment 3
[0044] N-[3-((5-sulfamoyl-1,3,4-thiadiazol-2-yl)amino)-3-oxopropyl]pyropheophytin (II 2 ) preparation:
[0045]
[0046] Compound II was prepared by the synthetic method of compound I 2 . 1 H NMR (600MHz, DMSO-d 6 )δ: 13.00(s,1H), 9.65(s,1H), 9.38(s,1H), 8.88(s,1H), 8.27(s,2H), 8.18(dd,J=17.8,11.6Hz,1H ),8.07(s,1H), 6.36(d,J=17.9Hz,1H),6.19(d,J=11.6Hz,1H),5.21(s,1H),5.13(s,1H),4.53(d ,J=7.7Hz, 1H), 4.25(d, J=8.9Hz, 1H), 3.64(d, J=8.1Hz, 2H), 3.59(s, 3H), 3.42(s, 3H), 3.41(s ,1H),3.17(s,3H),2.68(d,J=7.2Hz,2H),2.61(q,J=5.5,4.9Hz,1H),2.38(s,2H),2.12-2.08(m, 2H), 1.74 (d, J=7.4Hz, 3H), 1.59(s, 3H), 0.18(s, 1H), -2.03(s, 1H). 13 C NMR (151MHz, DMSO-d 6 )δ: 195.30,172.07,170.73,164.29,161.46,161.12,147.82,144.34,140.57,136.95,135.59,134.71, 131.52,128.92,122.57,105.89,103.76,96.20,93.63,51.19,49.40,47.52,35.26,34.54 , 32.32, 30.21, 22.83, 18.40, 17.34, 11.92, 11.52, 10.61. HRMS (MALDI-TOF) m / z: calcd for C 38 h 42 N 9 o 5 S 2 [M] + 768.27448,found 768.27272.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com