Preparation method of p-guanidinobenzoic acid hydrochloride
A technology of guanidinobenzoic acid hydrochloride and p-aminobenzoic acid, which is applied in the field of preparation of p-guanidinobenzoic acid hydrochloride, can solve the problems of cumbersome handling, high risk and high risk of using raw materials, and achieve The effect of high raw material safety, strong experimental operability, and short route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
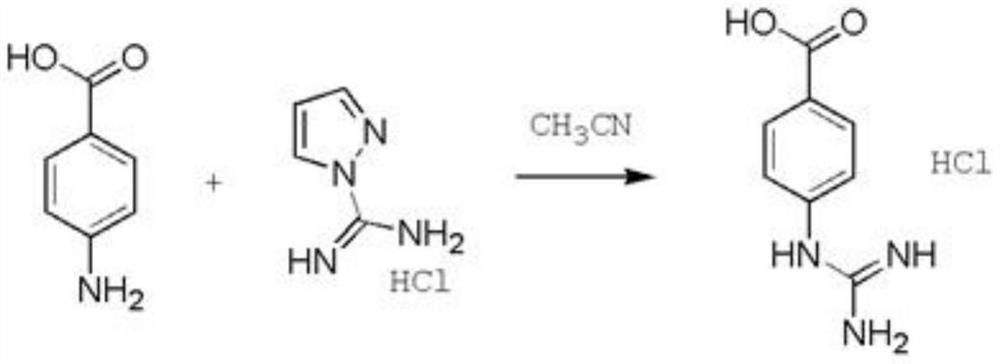
[0023] Preparation method of hydrochloride saline hydrochloride, according to an embodiment of the present invention, including:
[0024] The anti-aminobenzoic acid was refluxed to a pyrazole hydrochloride to form the hydrochloride salt of guanidylbenzoic acid.
[0025] That is, the preparation method of the hydrochloride salt of the hydrochloride of the present invention is selected to form a refluxed reaction in the preparation of amino benzoic acid and pyrazole hydrochloride. Hydrochloride.
[0026] The specific reaction process is as follows:
[0027]
[0028] Further, the molar ratio of amino benzoic acid to the pyrazole hydrochloride, for example, 1: (0.8-1.5). Among them, excessive pyrazole hydrochloride is preferably used to promote the reaction, improve the yield. Preferably, the molar ratio of amino benzoic acid to pyrazole hydrochloride has 1: 1.2.
[0029] Among them, the solvent used in the reflux reaction can be, for example, any of acetonitrile, toluene, dichlorom...
Embodiment 1
[0035] EtOAc The hour reaction is over.
[0036] The reaction solution was concentrated to evaporated to evaporated to 90 mL of water, stirred with solid, stir filtered, filter cake was washed over, collect filter cake, dried 70 ° C to give 42.27 g of thrombipanoic acid hydrochloride, 89.5%.
[0037] The reactant was used to confirm the product structure, the data is as follows:
[0038] 1H NMR (Model: Avance III HD 400M, CDCL3, 400MHz): δ = 12.98 (S, 1H), 10.51 (S, 1H), 7.98-7.96 (D, 2H), 7.82 (S, 4H), 7.34-7.32 (D, 2H), the test results are consistent with the structure.
Embodiment 2
[0040] 1 l of reaction bottles were added to aminobenzoic acid (182 g, 1.327 mol, 1.0 eq), pyrazole hydrochloride (233.43 g, 1.593 mol, 1.2eq) to add acetonitrile (550 mL, 3P), heated to 82 ° C back 3 The hour reaction is over.
[0041] The reaction liquid was concentrated to evaporase, cocked 550 ml of water, stirred and stirred with solid, washed with water, and the filter cake was washed once, and dried 70 ° C, to give 261.26 g of guanidylpanoic acid hydrochloride, yield 91.3%.
[0042] The reactant was used to confirm the product structure, the data is as follows:
[0043] 1H NMR (Model: Avance III HD 400M, CDCL3, 400MHz): δ = 12.98 (S, 1H), 10.50 (S, 1H), 7.99-7.96 (D, 2H), 7.82 (S, 4H), 7.34-7.32 (D, 2H), the test results are consistent with the structure.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com