Method for detecting pitavastatin calcium intermediate and impurities
A technology of pitavastatin calcium and detection method, which is applied in the field of drug detection and analysis, and can solve disadvantages and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
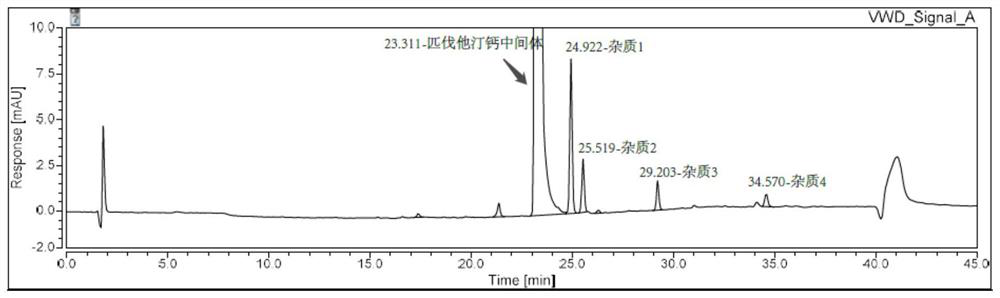
Embodiment 1
[0040] Instrument: Agilent 1260
[0041] Chromatographic column: waters Symmetry C18 4.6×150mm, 3.5um
[0042] Mobile phase: Phase A: 10mmol / L potassium dihydrogen phosphate aqueous solution (phosphoric acid to adjust pH=3.2)
[0043] Phase B: Acetonitrile
[0044] Detection wavelength: 245nm
[0045] Flow rate: 1.0ml / min
[0046] Column temperature: 25°C
[0047] Injection volume: 10μl
[0048] Table 1. Gradient elution program of Example 1
[0049]
[0050]
[0051] Diluent (blank solution): acetonitrile.
[0052] Preparation of impurity localization solution: take appropriate amount of reference substances of impurity 1, impurity 2, impurity 3, impurity 4 and impurity 5, weigh them accurately, add acetonitrile to dissolve and dilute, and make the contents of impurity 1-5 in each 1ml are about Solutions of 0.9 μg, 0.45 μg, 0.3 μg, 0.1125 μg, and 0.3 μg were used as positioning solutions for impurities 1-5, respectively.
[0053] System suitability solution prep...
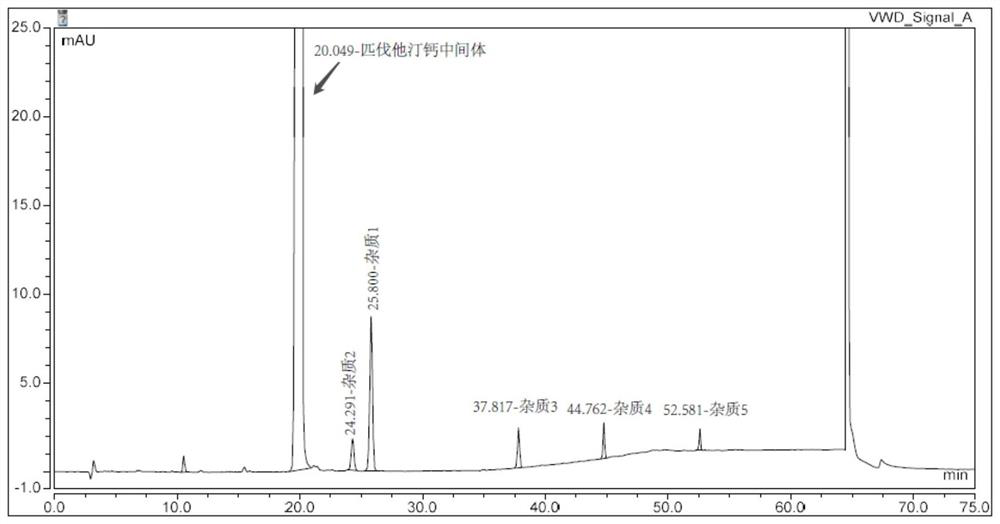
Embodiment 2
[0058] Instrument: Agilent 1260
[0059] Chromatographic column: SVEA phHex 4.6×250mm, 3.5um
[0060]Mobile phase: Phase A: 5mmol / L ammonium acetate buffer solution (adjust pH to 3.2 with phosphoric acid)
[0061] Phase B: Acetonitrile
[0062] Detection wavelength: 245nm
[0063] Flow rate: 0.8ml / min
[0064] Column temperature: 25°C
[0065] Injection volume: 10μl
[0066] Table 2. Gradient elution program of Example 2
[0067] Elution time (min) Phase A (%) Phase B (%) 0 55 45 5 55 45 30 45 55 45 10 90 60 10 90 60.1 55 45 75 55 45
[0068] Adopt the same method as Example 1 to prepare impurity positioning solution, system suitability solution, wherein the consumption of intermediates and impurities is shown in Table 3 below, adopt the same sample determination method as Example 1 to record the retention time of intermediates and impurities Also shown in Table 3 below.
[0069] Table 3. The positioning detect...
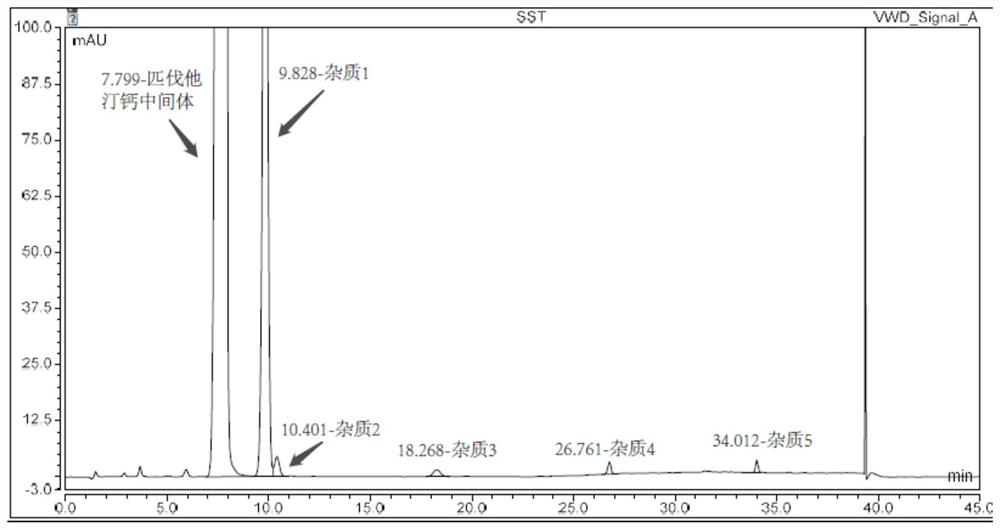
Embodiment 3
[0073] Instrument: Agilent 1260
[0074] Chromatographic column: Agilent ZORBAX SB-C8 4.6×150mm, 5μm;
[0075] Mobile phase: Phase A: 5mmol / L ammonium acetate buffer solution (adjust pH to 3.2 with phosphoric acid)
[0076] Phase B: Acetonitrile
[0077] Detection wavelength: 245nm
[0078] Flow rate: 1.0ml / min
[0079] Column temperature: 25°C
[0080] Injection volume: 10μl
[0081] Table 4. Gradient elution program of Example 3
[0082] Elution time (min) Phase A (%) Phase B (%) 0 55 45 5 55 45 20 50 50 30 10 90 37 10 90 37.1 55 45 45 55 45
[0083] Adopt the same method as Example 1 to prepare impurity localization solution, system suitability solution, wherein the consumption of intermediate and impurity is shown in Table 5 below, adopt the same sample determination method as Example 1 to record the retention time of intermediate and impurity Also shown in Table 5 below.
[0084] Table 5. The positioning d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Volume fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com