Method for producing nitric acid and sodium nitrate by using nuclear-grade hafnium oxide production discharged wastewater
A hafnium dioxide and sodium nitrate technology, which is applied in the purification of nitrogen oxides/oxyacids, nitric acid, and alkali metal nitrates, can solve the problems of inability to recover nitric acid and consume large amounts of liquid alkali, and achieve full resource utilization and low cost Effects of low and obvious social and economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
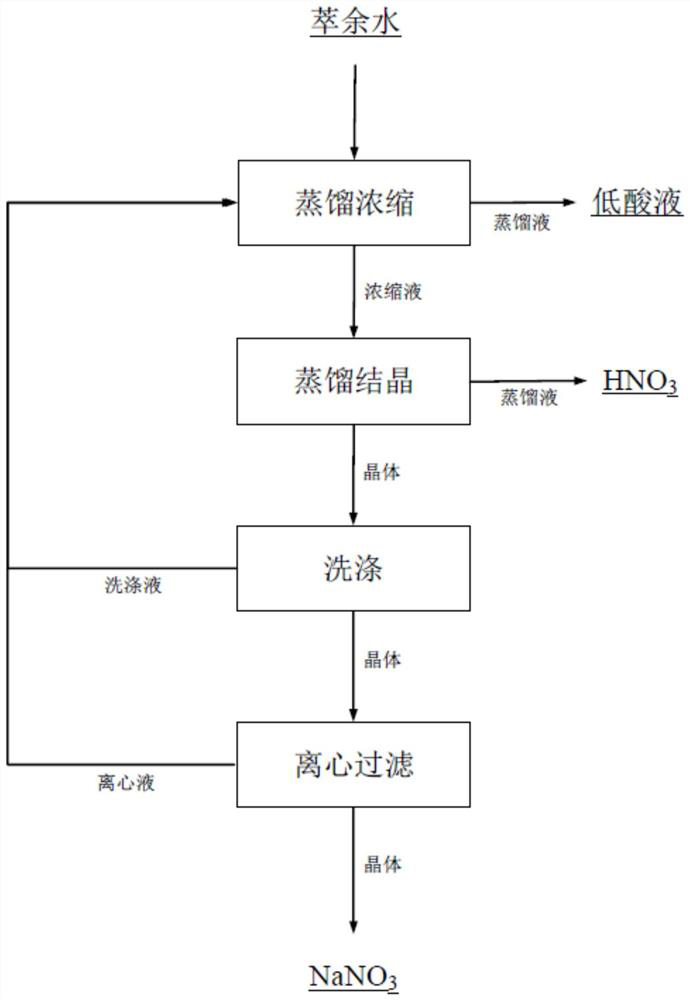
[0028] (1) The 195L raffinate produced by the enrichment of hafnium during the production of nuclear-grade hafnium dioxide was sampled and analyzed for a nitric acid concentration of 3.95mol / L; Na + The concentration is 49.75 (g / L), and it is transported to the preheater with a pump. After the feed liquid is preheated at 85°C, it enters the first-effect evaporator. The specific gravity of the concentrated solution is 1.265.
[0029] (2) The concentrate is pumped into the second-effect evaporation crystallizer, the temperature of the second-effect evaporation crystallizer is controlled at 80°C, the pressure is controlled at 12kpa for distillation and crystallization, and the second-effect distillate nitric acid is collected to obtain a concentration of 6.02mol / L Nitric acid 103L.
[0030] (3) The sodium nitrate particles evaporated and crystallized enter the centrifuge under the action of gravity and are washed once with the zirconium precipitation mother liquor with a solid-t...
Embodiment 2
[0032] (1) The 206L raffinate produced by the enrichment of hafnium during the production of nuclear-grade hafnium dioxide was sampled and analyzed for a nitric acid concentration of 3.93mol / L; Na + The concentration is 49.67 (g / L), and it is pumped to the preheater. After the feed liquid is preheated at 85°C, it enters the first-effect evaporator. The temperature of the first-effect evaporator is controlled at 115°C, and the pressure is controlled at 60kpa. The specific gravity of the concentrated solution is 1.261.
[0033] (2) The concentrated solution is pumped into the second-effect evaporation crystallizer, the temperature of the second-effect evaporation crystallizer is controlled at 75°C, the pressure is controlled at 10kpa for distillation and crystallization, and the second-effect distillate nitric acid is collected to obtain a concentration of 6.06mol / L Nitric acid 109L.
[0034] (3) The sodium nitrate particles evaporated and crystallized enter the centrifuge unde...
Embodiment 3
[0036] (1) The 200L raffinate produced by the enrichment of hafnium during the production of nuclear-grade hafnium dioxide was sampled and analyzed for the concentration of nitric acid to be 3.96mol / L; Na + Concentration is 49.73 (g / L), pumped to the preheater, the feed liquid is preheated at 85°C and enters the first-effect evaporator, the temperature of the first-effect evaporator is controlled at 120°C, and the pressure is controlled at 55kpa for distillation and concentration, concentrated to The specific gravity of the concentrated solution is 1.267.
[0037] (2) The concentrated solution is pumped into the second-effect evaporation crystallizer, the temperature of the second-effect evaporation crystallizer is controlled at 85°C, the pressure is controlled at 15kpa for distillation and crystallization, and the second-effect distillate nitric acid is collected to obtain a concentration of 6.10mol / L Nitric acid 105.5L.
[0038] (3) The sodium nitrate particles evaporated a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com