Analysis method of vernakalant related substances
An analysis method and technology related to substances, applied in the field of chemical analysis, can solve the problems of relatively large and difficult detection and separation, achieve optimal separation and improve the effect of symmetry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Instruments and conditions: Waters e2695 high performance liquid chromatography
[0053] Chromatographic column: cellulose-tris((3,5-dichlorophenylcarbamate) chiral column DAICEL 4.6mm×250mm, 3μm);
[0054] Mobile phase volume ratio: the ratio of n-hexane-absolute ethanol-trifluoroacetic acid is 86:14:0.1;
[0055] Detection wavelength: 210nm;
[0056] Column temperature 30°C;
[0057] Flow rate: 1.0ml / min;
[0058] Injection volume: 10 μl.
[0059] Experimental steps:
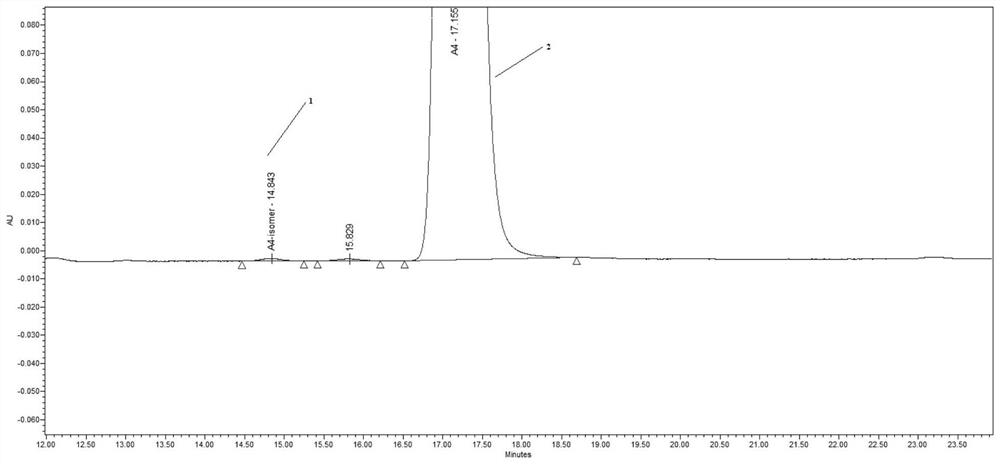
[0060] Vernakalant intermediate A4: Accurately weigh an appropriate amount of Vernakalant intermediate A4 reference substance, dissolve it with the mobile phase, dilute it with the mobile phase to a solution containing about 2 μg per 1 ml, accurately measure 10 μl and inject it into the chromatograph, record Chromatogram, Vernakalant intermediate A4 chromatogram see figure 1 , the peak was at 17.098min.
[0061] Vernakalant intermediate A4 enantiomer: Accurately weigh an appropriate amount of t...
Embodiment 2
[0064] Instruments and conditions: Waters e2695 high performance liquid chromatography
[0065] Chromatographic column: cellulose-tris((3,5-dichlorophenylcarbamate) chiral column DAICEL 4.6mm×250mm, 3μm);
[0066] Mobile phase volume ratio: the ratio of n-hexane-absolute ethanol-trifluoroacetic acid is 85:15:0.1;
[0067] Detection wavelength: 210nm;
[0068] Column temperature 30°C;
[0069] Flow rate: 1.0ml / min;
[0070] Injection volume: 10 μl.
[0071] Test steps and results:
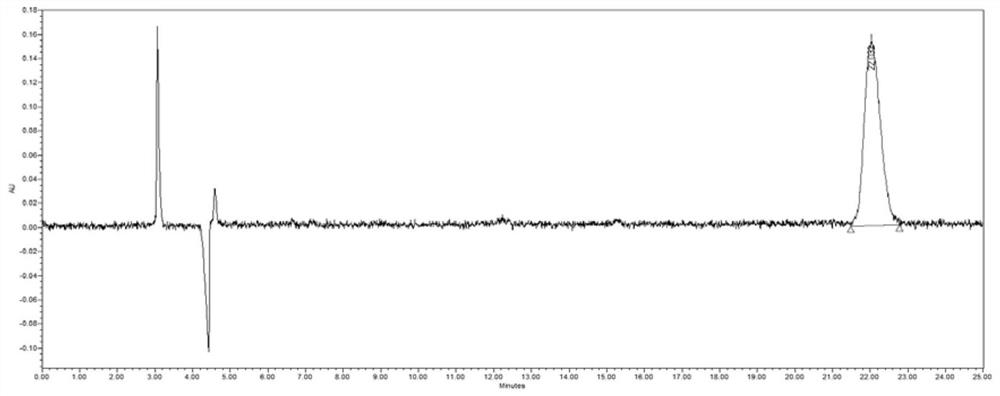
[0072] Precisely measure 10 μl of the test solution, inject it into the liquid chromatograph, record the chromatogram, see Figure 4 , the elution times of vernakalant intermediate A4 and its isomers were 15.571 min and 14.155 min, respectively, the separation degree of the two was 3.0, and the symmetry was good. Vernakalant intermediate A4 and its enantiomers were completely separated under these chromatographic conditions.
Embodiment 3
[0074] Instruments and conditions: Waters e2695 high performance liquid chromatography
[0075] Chromatographic column: cellulose-tris((3,5-dichlorophenylcarbamate) chiral column DAICEL 4.6mm×250mm, 3μm);
[0076] Mobile phase volume ratio: the ratio of n-hexane-absolute ethanol-trifluoroacetic acid is 95:5:0.1;
[0077] Detection wavelength: 210nm;
[0078] Column temperature 30°C;
[0079] Flow rate: 1.0ml / min;
[0080] Injection volume: 10 μl.
[0081] Test steps and results:
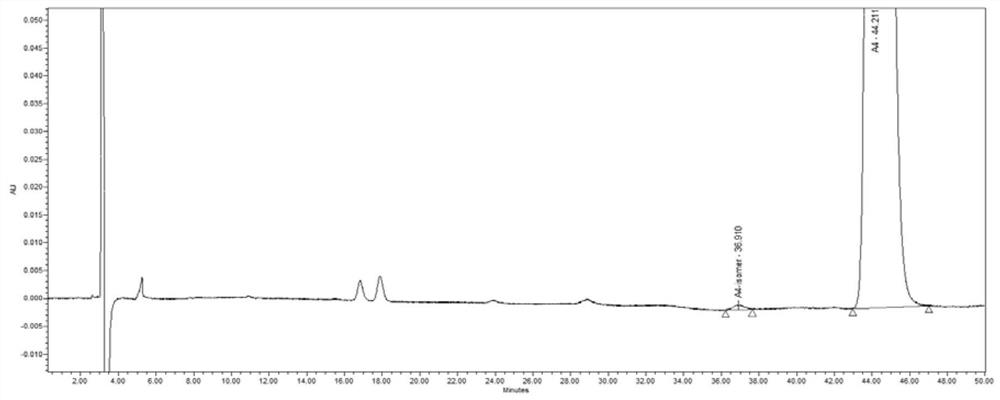
[0082] Precisely measure 10 μl of the test solution, inject it into the liquid chromatograph, record the chromatogram, see Figure 5 , the elution times of vernakalant intermediate A4 and its isomers were 44.211min and 36.910min respectively, the separation degree of the two was 5.7, and the symmetry was good. Vernakalant intermediate A4 and its enantiomers were completely separated under these chromatographic conditions.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com