Indapamide solid dispersion and preparation method thereof
A technology of solid dispersion and indapamide, which can be applied to medical preparations without active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc., and can solve the problems of organic solvent residues in indapamide solid dispersions. , to achieve the effects of easy control of operating conditions, improved dissolution performance, and overcoming the problem of organic solvent residues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Single factor investigation of the influence of each factor on the recovery rate and drug loading of indapamide solid dispersion or the cumulative dissolution rate within 60min
[0043] Single factor experiment: the effect of solvent type on the recovery rate of indapamide solid dispersion
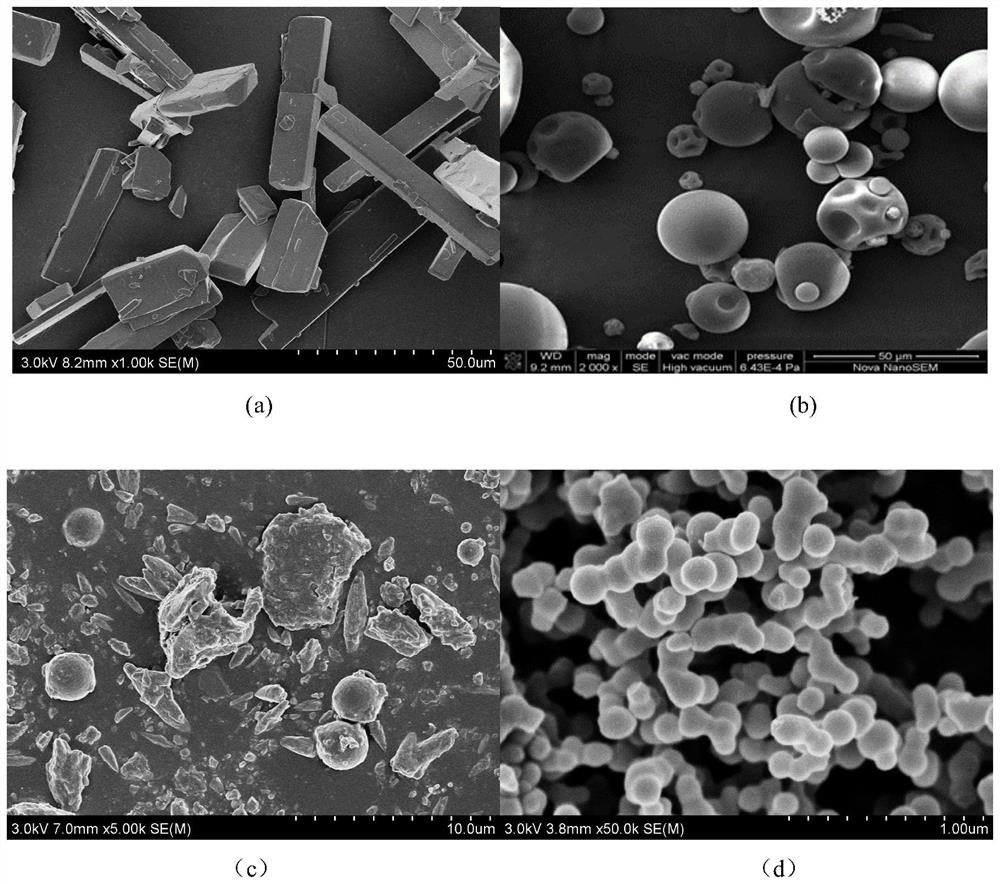
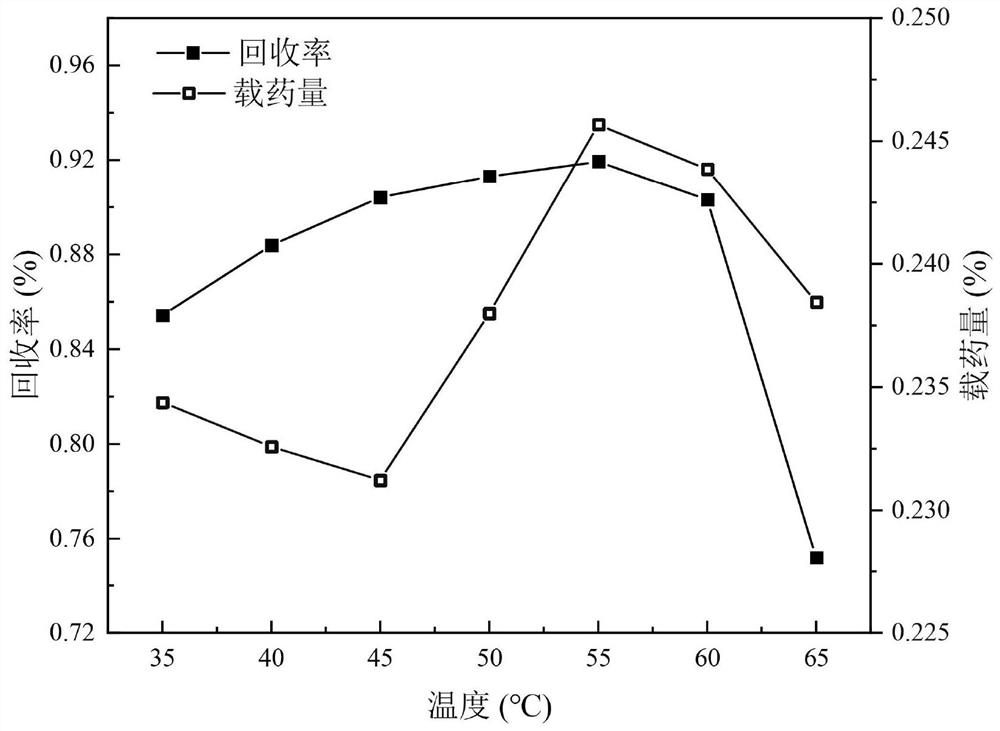
[0044] Investigate ethanol, acetone, dichloromethane, acetone:dichloromethane=3:2, acetone:dichloromethane=1:1, acetone:dichloromethane=2:3 solvent respectively, to indapamide solid dispersion Effect of recovery and drug loading. Other parameters are that the ratio of drug to carrier is 1:2, the crystallization pressure is 13MPa, the crystallization temperature is 43°C, the solution volume flow rate is 1.0mL / min, the solution mass concentration is 5mg / mL, CO 2 The flow rate is (3.0±0.2) L / min. Since PVP-K30 is poorly soluble in acetone, acetone is firstly excluded as a solvent, and most samples of indapamide in ethanol are formless viscous liquids. When using dichlorom...
Embodiment 2
[0055] Embodiment 2: Orthogonal optimization optimal test factor parameter
[0056] Orthogonal experimental design and results
[0057] With recovery rate as index, design orthogonal experiment to investigate crystallization pressure (A), crystallization temperature (B), solution volume flow rate (C), solution mass solubility (D), table 1 is factor level design table, and table 2 is positive Submit the experimental design and results.
[0058] Table 1 Factor level table
[0059]
[0060] Table 2 Orthogonal design and results
[0061]
[0062] Table 3 ANOVA results
[0063]
[0064]
[0065] f 0.05 (2, 2) = 19.00
[0066] The direct weighting method in the comprehensive scoring method is used for single-index integration. Under the same experimental conditions, the weight sum of the two indicators is 1. The recovery rate is the main indicator, with a weight of 0.6 and a weight of drug loading of 0.4. According to the formula, comprehensive score = recovery rate...
Embodiment 3
[0069] Example 3: Preparation of Indapamide Solid Dispersion Using Optimal Process Conditions
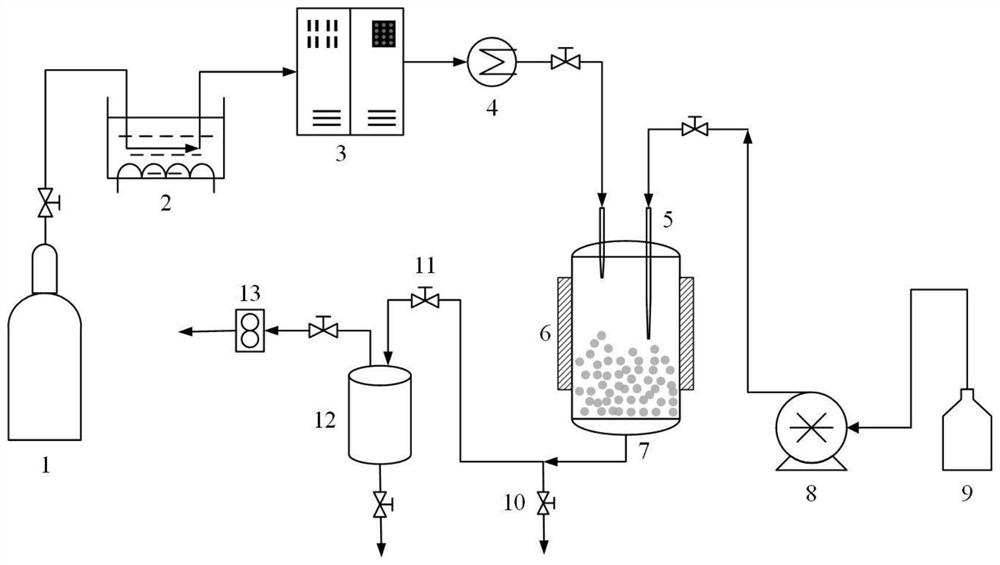
[0070] Application of supercritical fluid enhanced solution dispersion technology to prepare indapamide solid dispersion comprises the following steps:
[0071](1) Preparation of indapamide solution: take the indapamide bulk drug and the PVP-K30 carrier and dissolve them in an organic solvent according to the ratio of the drug to the carrier as 1: 3 to obtain the indapamide-carrier solution, the solution mass The concentration is 3 mg / mL; wherein, the organic solvent is acetone:dichloromethane=1:1.
[0072] (2) CO 2 Pass into the crystallization kettle at 3.0±0.2L / min, adjust the temperature in the crystallization kettle to 50°C, and the pressure to 19MPa;
[0073] (3) Continue to feed CO 2 , maintain the constant temperature and pressure in the crystallization tank, while the indapamide solution prepared in step (1) is sprayed into the crystallization tank from the top of the cr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com