Application of harringtonine in preparation of medicine for resisting novel coronavirus
A technology of harringtonine and antiviral drug, which is applied in the field of antiviral drug preparation and achieves significant anti-SARS-CoV-2 effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 3
[0030] Example 1 Harringtonine specifically binds to the S protein of SARS-CoV-2 and the cell TMPRSS2 protein
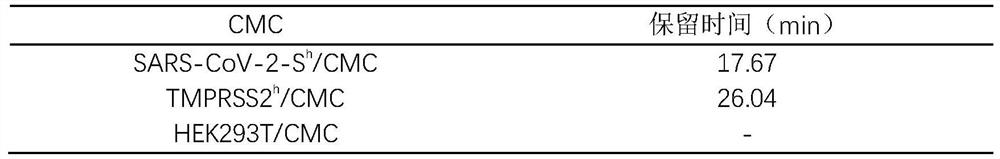
[0031] CMC is a kind of affinity chromatography, which uses the cell membrane with specific receptor overexpression as the stationary phase, which can realize high-throughput screening of components that specifically bind to membrane receptors under biomimetic conditions, and is a reliable means for screening active ingredients of traditional Chinese medicine. The S protein of SARS-CoV-2 and HEK293T cells with high expression of cell TMPRSS2 enzyme were used to prepare cell membranes, which were adsorbed with activated silica gel to prepare cell membrane chromatography stationary phases, and SARS-CoV-2-S were obtained after wet packing. h / CMC column and TMPRSS2 h / CMC chromatographic column for HPLC detection, and HEK293T / CMC is the CMC control for protein specific binding. The results are shown in Table 1:
[0032] Table 1 Retention time of harringtonine on vario...
Embodiment 2 3
[0035] Example 2 Harringtonine inhibits cell membrane fusion induced by binding of S protein to ACE2
[0036] ACE2 high-expressing cells were seeded in 96-well plates, and each well was supplemented with medium to 50 μL. Incubate for 2 hours in a 37°C, 5% CO2 incubator to adhere to the wall. Add harringtonine prepared in culture medium at a concentration of 0, 0.625, 1.25, 2.5, 5, and 10 μM, and add 50 μL of HEK293T cells highly expressing S protein and green fluorescent protein (GFP), and continue to infect for 8 hours. , for fluorescence microscopy observations. It can be seen that the cells with high expression of ACE2 in the non-administration group and the HEK293T cells with high expression of S protein and GFP undergo membrane fusion to form syncytia of multiple cells. When the drug concentration of harringtonine in the administration group reached 1 μM, the number of syncytia was significantly reduced, and the boundaries between individual cells were clear. It shows ...
Embodiment 3 3
[0037] Example 3 Harringtonine inhibits SARS-CoV-2 pseudovirus from infecting ACE2 high-expressing cells
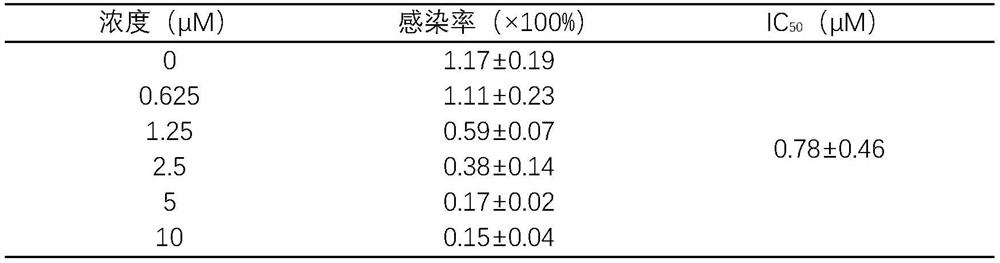
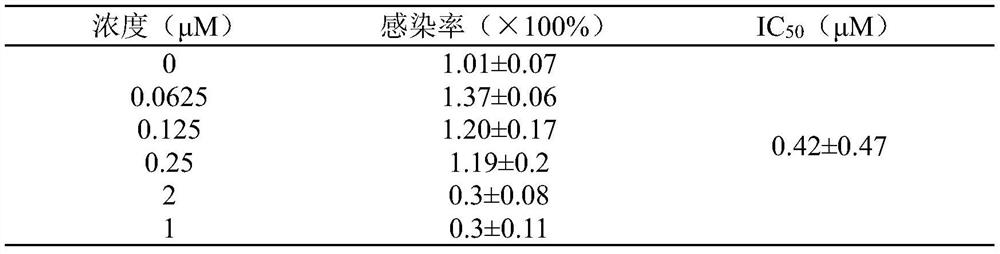
[0038] ACE2 high-expressing cells were seeded in 96-well plates, and each well was supplemented with medium to 50 μL. 37°C, 5% CO 2 Incubate in the incubator for 2 hours to adhere to the wall. Add different concentrations of harringtonine prepared in culture medium, add 5 μL SARS-CoV-2 pseudovirus to each well and infect for 4 hours, make up to 100 μL culture volume, continue to infect for 6-8 hours, replace with 200 μL new complete culture Then continue to culture at 37°C for 48h. The Luciferase Assay System kit detects the luminescence value of Luciferase. See Table 2 for the results:
[0039] Table 2 Harringtonine inhibits SARS-CoV-2 pseudovirus from infecting ACE2 high-expressing cells
[0040]
[0041] As can be seen from Table 2, harringtonine has inhibitory effect on the infectivity of SARS-CoV-2 pseudovirus in a dose-dependent manner, and its IC 50 is 0.78±...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com