Thyroid stimulating hormone receptor antigen reagent and thyroid stimulating hormone receptor antibody quantitative detection kit
A thyroid stimulating hormone and kit technology, which can be used in biological tests, measuring devices, material inspection products, etc., and can solve problems such as interference and complex labeling processes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] A human thyroid-stimulating hormone receptor antibody quantitative detection kit, comprising: magnetic particles coated with anti-human TSHR mouse monoclonal antibody A, antigen reagent (anti-human TSHR mouse monoclonal antibody B-recombinant human TSHR complex), Sample diluent, horseradish peroxidase-labeled human stimulating thyrotropin receptor antibody and stimulating thyrotropin receptor antibody serial calibrator, the preparation process is as follows:
[0058] (1) Preparation of magnetic particle suspension coated with anti-human TSHR mouse monoclonal antibody A:
[0059] The carboxyl magnetic microspheres were activated under acidic conditions with EDC and NHS, the activation buffer was 0.1M MES (2-(N-morpholino)ethanesulfonic acid) buffer, and the activation time was 30min. After the activation was completed, Under the action of a magnetic field, the carboxyl magnetic microspheres are separated from the liquid, the supernatant is discarded, and an appropriate a...
Embodiment 2
[0078] Embodiment 2 stability evaluation
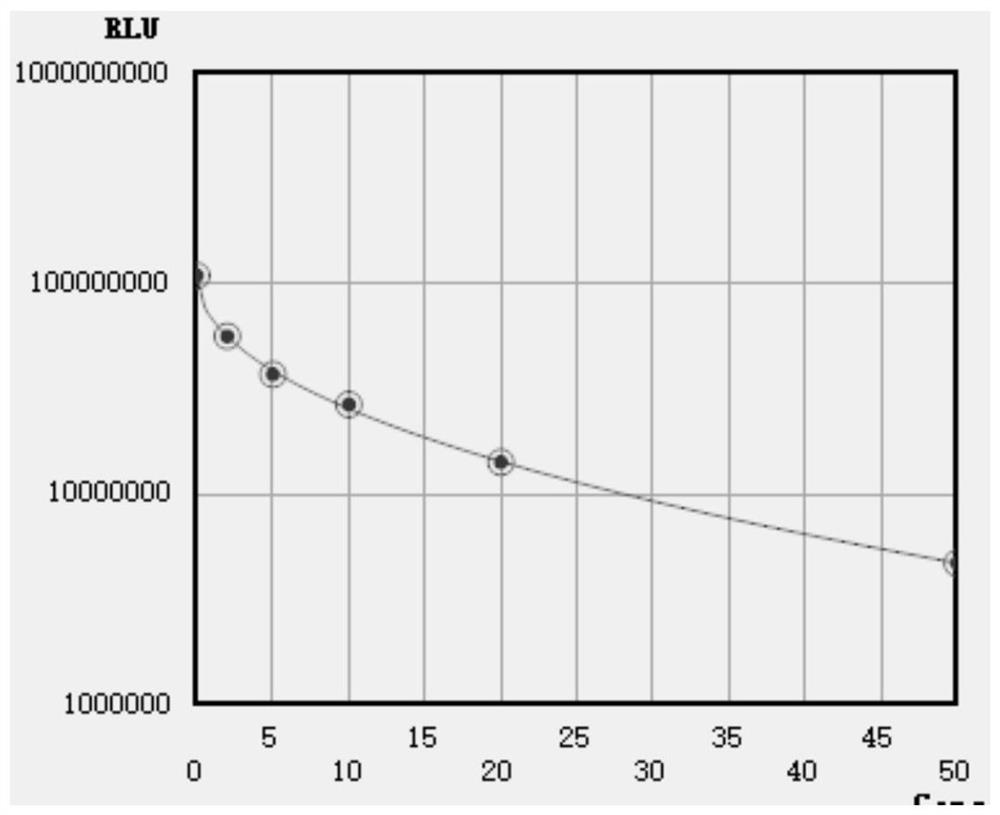
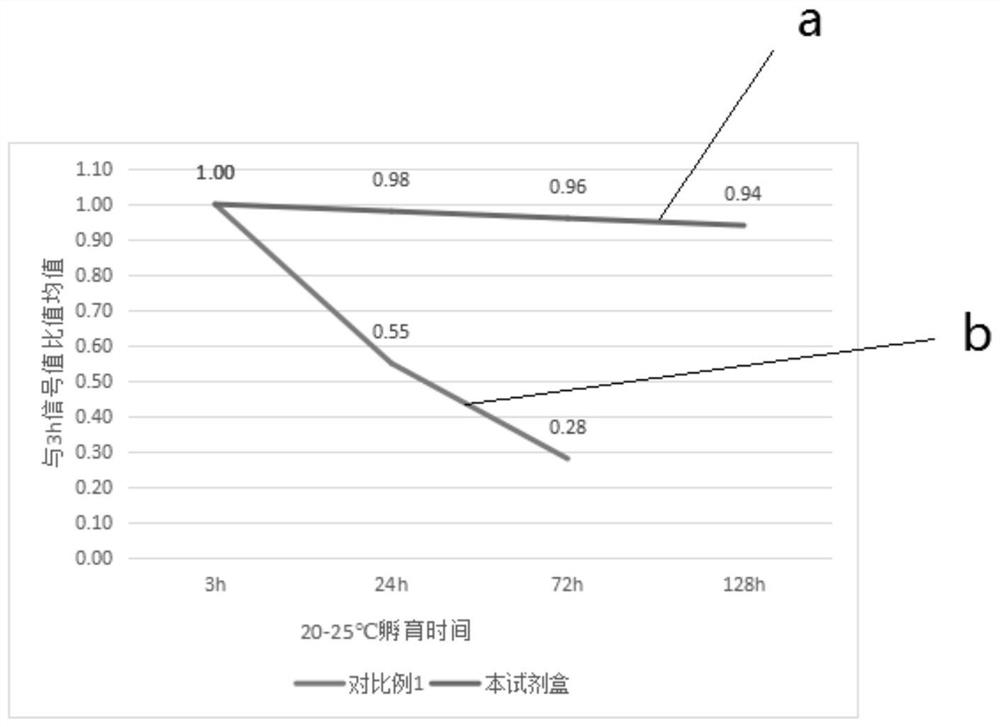
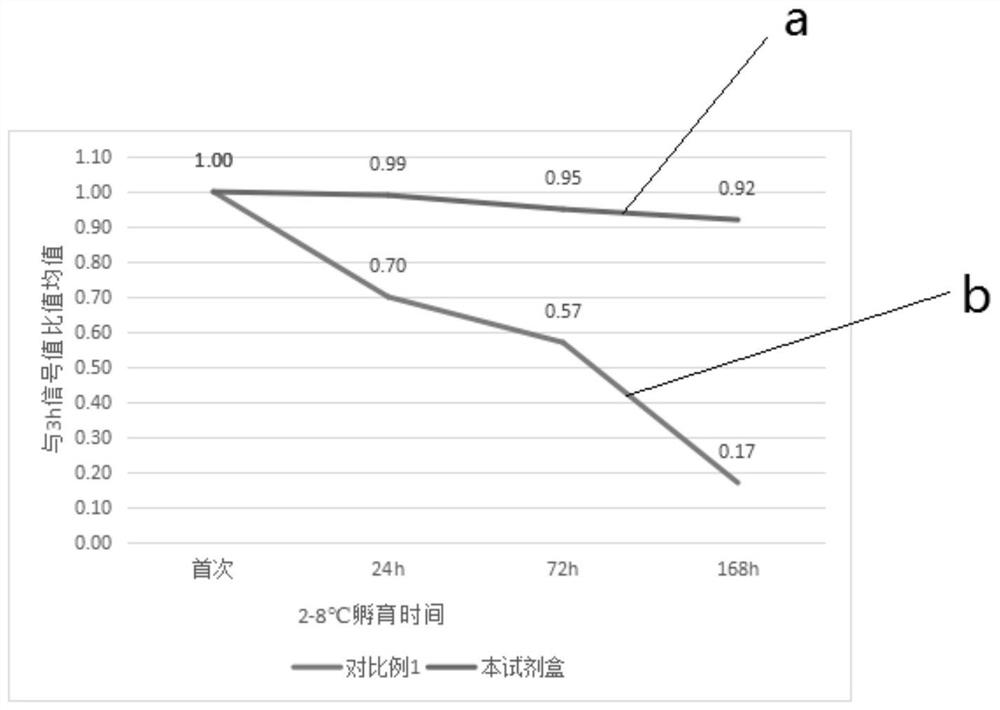
[0079] Stability evaluation method: using the kits provided in Comparative Example 1 and Example 1 to detect in parallel a series of stimulating thyrotropin receptor antibody concentrations 0IU / L, 2IU / L, 5IU / Calibrator of L, 10IU / L, 20IU / L, 50IU / L is measured on the automatic chemiluminescence instrument A2000 Plus system according to the detection steps, and the luminescence value of each group is obtained, and the signal value drop ≤ 10% is regarded as stable Good performance, the results are shown in Table 1, figure 2 , image 3 and Figure 4 , Table 1 is the stability evaluation result of the kit that the embodiment of the present invention and comparative example provide, figure 2 It is the stability evaluation result of the antigen reagent provided in the examples and comparative examples of the present invention at 20-25°C in the lyophilized solution, wherein curve a is the stability evaluation result of the antigen reage...
Embodiment 3
[0085] Embodiment 3 kit comparison
[0086] Quantitative detection kit for human thyroid stimulating hormone receptor antibody constructed according to Example 1 and kit C: quantitative detection kit for thyroid stimulating hormone receptor antibody (electrochemiluminescence method), kit D: human thyroid stimulating hormone receptor antibody The detection kit (enzyme-catalyzed chemiluminescence method) is used for detection and comparison of clinical samples, and the repeatability and interference of these three detection and quantification kits are analyzed and compared at the same time.
[0087] The detection principle of kit C is: competition method, magnetic particles coated with streptavidin, human immunocomplexes labeled with soluble porcine TSHR and biotinylated anti-porcine TSHR C-terminal mouse monoclonal antibody and ruthenium complex Monoclonal autoantibody M22 was tested;
[0088] The detection principle of kit D is: competition method, using FITC antibody to coat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com