Marfan syndrome detection kit based on FBN1 gene insertion mutation
A detection kit and insertion mutation technology, which are used in biological testing, microbial determination/inspection, measurement devices, etc., can solve problems such as large individual differences and difficulty in early diagnosis, achieve good accuracy, easy operation, and reduce disease Rate and Mortality Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1. FBN1 gene c.5688-5689 (p.Arg1897Glufs) insertion mutation detection kit The components of the kit described in Example 1 are described in Table 1 below.
[0042] Table 1. Kit components
[0043]
Embodiment 2
[0044] Example 2. Patient / Carrier Verification Experiment
[0045] 1. Sample collection and clinical data collection
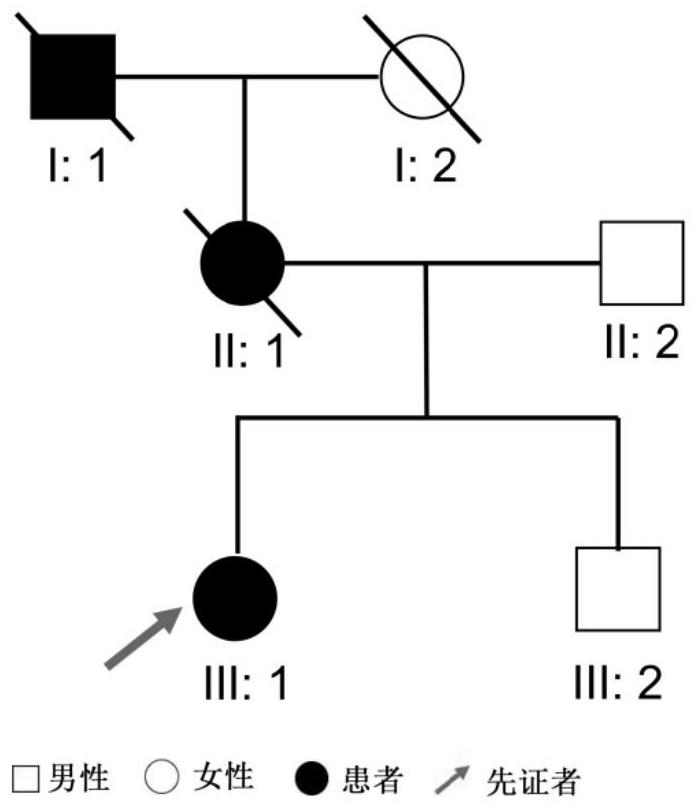
[0046] On the premise that the proband and his family members voluntarily signed the informed consent form, about 5mL whole blood samples were collected in Shenzhen Eye Hospital and stored in low-temperature refrigeration conditions, and a medical record database was established to record in detail the proband’s condition, family status and other information . This study was approved by the Ethics Committee of Shenzhen Eye Hospital.
[0047] 2. Preparation of Genomic DNA
[0048] A commercially available DNA extraction kit was used to extract whole genome DNA from human whole blood EDTA anticoagulated samples, and to detect the concentration and purity of DNA.
[0049] 3. PCR primer design
[0050] According to the DNA sequence of the FBN1 gene in the NCBI database (https: / / www.ncbi.nlm.nih.gov / ), primers were designed upstream and downstream of the FBN1 g...
Embodiment 3
[0079] Example 3. Repeatability detection
[0080] The method described in Example 2 of the present invention was used to repeatedly test the blood samples of one Marfan syndrome patient and one healthy person three times, and the test results are shown in Table 5 below.
[0081] Table 5. Repeatability test results
[0082] sample patient healthy person the first time Positive Negative the second time Positive Negative the third time Positive Negative
[0083] The above detection results show that the sites and detection primers described in this application have good reproducibility.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com