Injection type skin filling composition as well as preparation method and application thereof
A composition and polymer technology, applied in the field of medicine, can solve the problems of insignificant immediate filling effect, poor dispersibility of PLA particles, and gel shift of polycaprolactone, and achieve controllable degradation time, narrow particle size distribution, Reduces subcutaneous nodules, redness, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
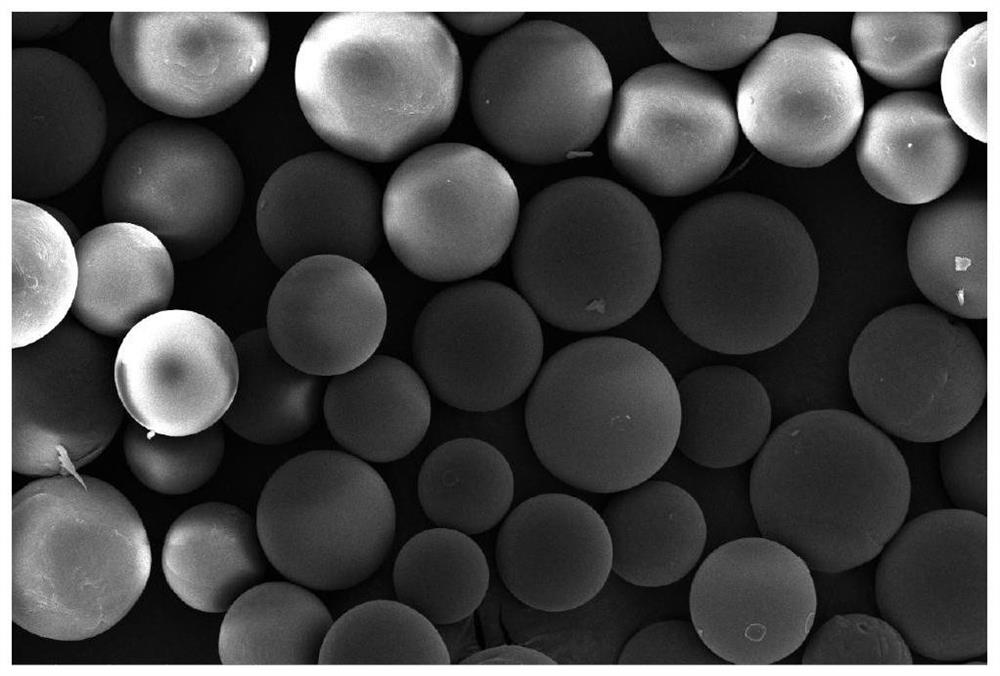
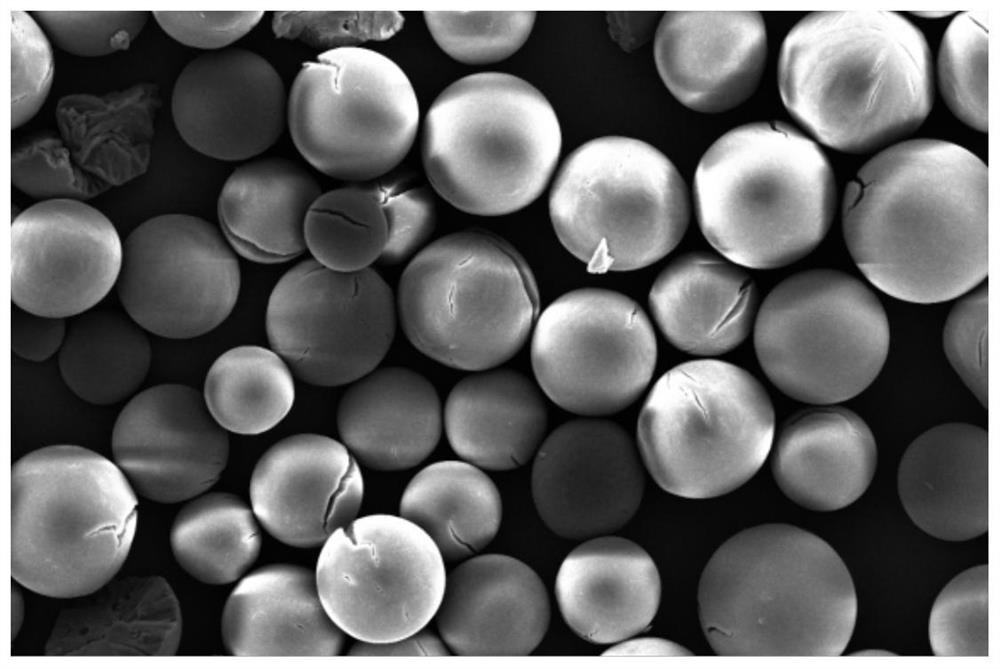
Image
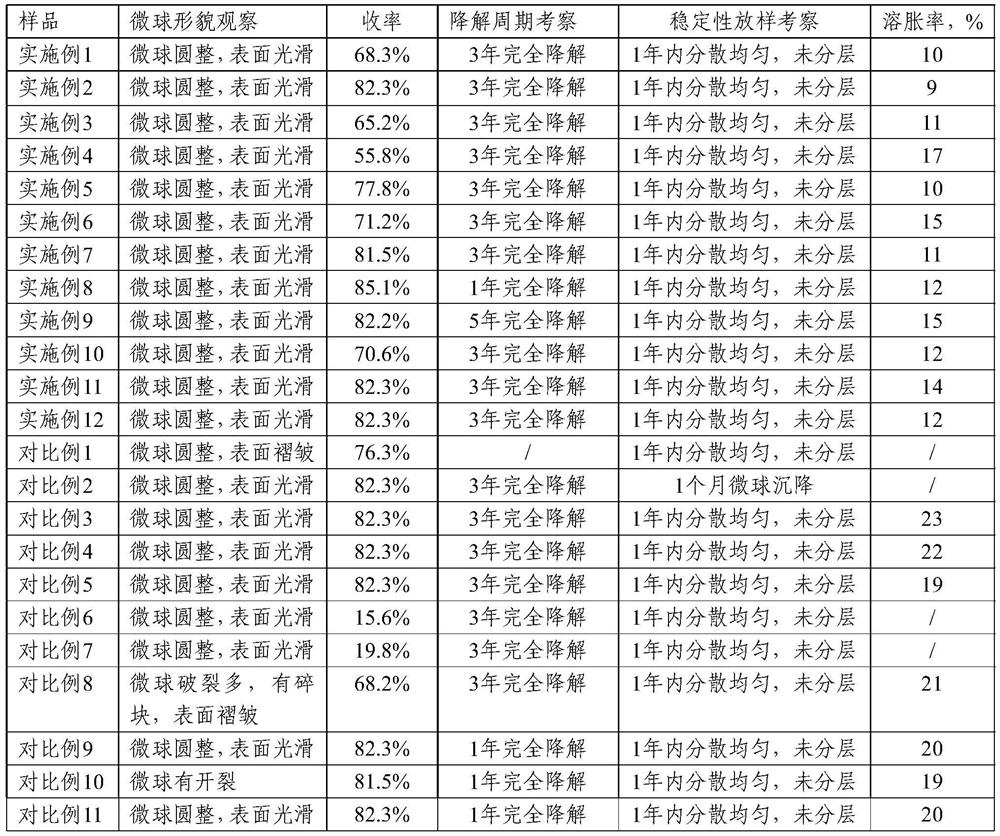
Examples
Embodiment 1
[0048] This experimental example provides a kind of preparation method of the composition of the present invention, specifically comprises the following steps:
[0049] Using tetrahydrofuran and dichloromethane as solvents (the volume ratio of the two solvents is 2:8), mix them with polycaprolactone (viscosity 1.2dl / g) to prepare an oil phase, wherein the mass fraction of polycaprolactone is 10% .
[0050] Add polyvinyl alcohol (viscosity 25mPa·s) and Tween-80 as solutes (the mass ratio of the two solutes is 9.5:0.5), add pure water, and configure an aqueous phase, wherein the solute mass fraction is 1%.
[0051] The volume ratio of oil phase to water phase is 1:5.
[0052] Preparation of microspheres by shear emulsification method: the specific parameter of mechanical stirring is 1000r / min, the oil phase is added dropwise in the water phase ice bath (5-8°C) and stirring is turned on, stirring and emulsifying for 30min, and then in Reduce the rotation speed at 5-8°C and cont...
Embodiment 2
[0060] This embodiment provides a preparation method of the composition of the present invention, which specifically includes the following steps:
[0061] Step 1: Preparation of microspheres
[0062] Using tetrahydrofuran and dichloromethane as solvents (the volume ratio of the two solvents is 2:8), mix them with polycaprolactone (viscosity 1.2dl / g) to prepare an oil phase, wherein the mass fraction of polycaprolactone is 10% .
[0063] Add polyvinyl alcohol (viscosity 25 mPa·s) and Tween-80 as solutes (the mass ratio of the two solutes is 9.5:0.5), add pure water, and configure an aqueous phase, wherein the solute mass fraction is 1%.
[0064] The volume ratio of oil phase to water phase is 1:5.
[0065] Preparation of microspheres by membrane emulsification method: the oil phase is passed through the SPG membrane (pore size is 10 μm) at a pressure of 0.2 kPa, and mixed with the water phase (under ice bath conditions of 5-8 °C) under stirring, and the stirring rate is 250...
Embodiment 3
[0073] This embodiment provides a preparation method of the composition of the present invention, which specifically includes the following steps:
[0074] Step 1: Preparation of microspheres
[0075] Using tetrahydrofuran and dichloromethane as solvents (the volume ratio of the two solvents is 1:9), mix them with polycaprolactone (viscosity 1.2dl / g) to prepare an oil phase, wherein the mass fraction of polycaprolactone is 10% .
[0076] Preparation of microspheres by spray drying method: put the oil phase into the spray drying equipment, and the specific setting parameters are: the inlet temperature is 65°C, the outlet temperature is 40°C, the feed rate is 10ml / min, and the high-pressure air flow rate is 400L / h . The particle size range of the obtained microspheres is 20-60 μm.
[0077] The microspheres obtained above were sieved to obtain microspheres with a particle size of 25-40 μm (accounting for 65.2% by mass of the total microspheres).
[0078] Step 2: Preparation o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com