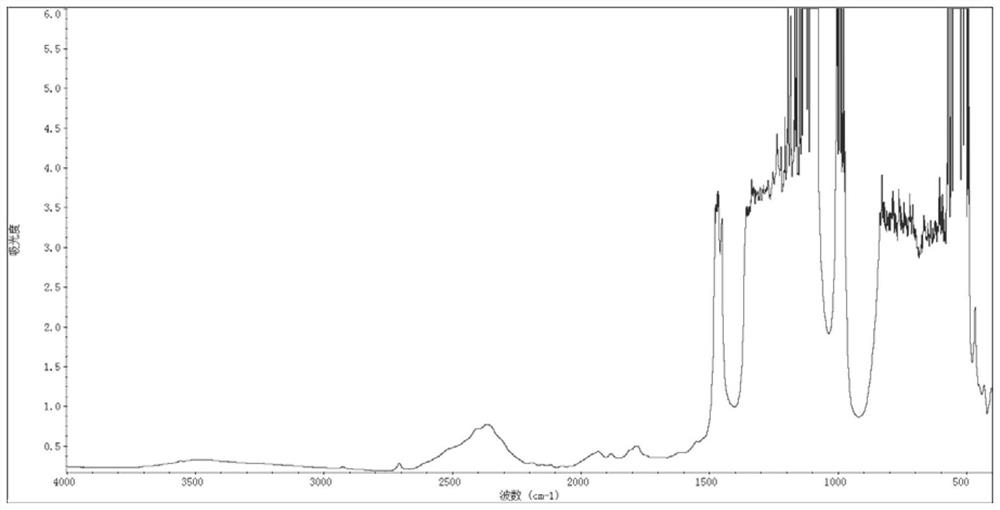
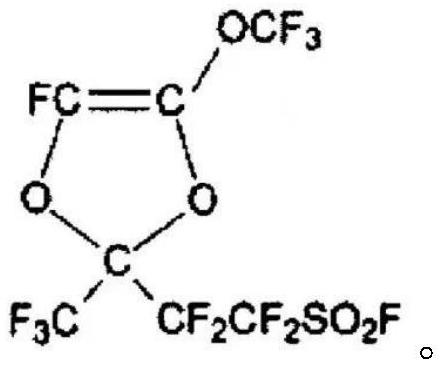
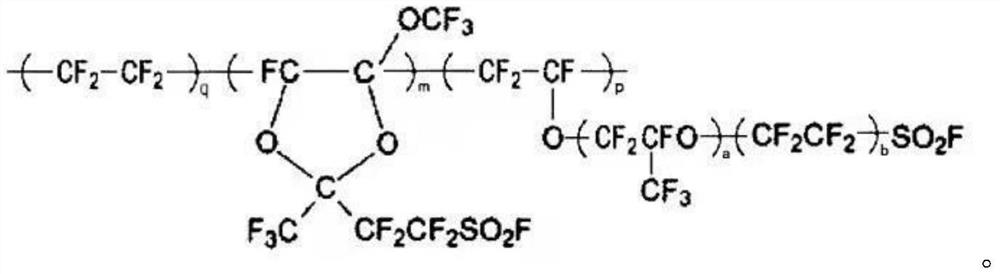
Bulk polymerization preparation method of perfluorinated sulfonic acid resin
A perfluorosulfonic acid resin, bulk polymerization technology, applied in the field of fuel cells, can solve the problems of difficult market promotion, high price, difficulty in synthesizing monomer A, etc., and achieves improved solubility, high affinity, improved The effect of machinability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] After cleaning and drying the reactor, vacuumize and replace with nitrogen until the water content is below 100ppm and the oxygen content is below 10ppm. Vacuumize and fill the tetrafluoroethylene monomer to 0.1MPa, then vacuumize to 0.0001MPa, and then fill 180g monomer CF 2 = CFOCF 2 CF(CF 3 )OCF 2 CF 2 SO 2 F, 168gCF 2 = CFOCF 2 CF 2 SO 2 F and 68g of monomer C were added to the reaction kettle, the temperature was raised to 60°C, tetrafluoroethylene was introduced until the pressure reached 2MPa, and 10mL containing 0.01g of perfluorobutyryl peroxide (CF 3 CF 2 CF 2 CO-OO-CCF 2 CF 2 CF 3 ) initiator solution, maintain the reaction pressure at about 2MPa, and continuously add 45g monomer CF 2 = CFOCF 2 CF(CF 3 )OCF 2 CF 2 SO 2 F and 8.5g monomer C, when the tetrafluoroethylene addition reaches 100g, stop adding, allow reaction to continue, when still internal pressure is reduced to 1MPa, stop reaction, reclaim unreacted tetrafluoroethylene monomer....
Embodiment 2
[0036] After cleaning and drying the reactor, vacuumize and replace with nitrogen until the water content is below 100ppm and the oxygen content is below 10ppm. Vacuumize and fill tetrafluoroethylene monomer to 0.1MPa, then vacuumize to 0.0001MPa, then fill 450g monomer CF 2 = CFOCF 2 CF(CF 3 )OCF 2 CF 2 SO 2 Add F and 21g of monomer C into the reactor, raise the temperature to 30°C, feed tetrafluoroethylene until the pressure reaches 1MPa, add 10mL of initiator solution containing 0.05g of diisopropyl peroxydicarbonate (IPP) with a metering pump, Maintain the reaction pressure at about 1MPa, and continuously add 4.5g monomer CF2 = CFOCF 2 CF(CF 3 )OCF 2 CF 2 SO 2 F and 8.5g monomer C, when the tetrafluoroethylene addition reaches 100g, stop adding, allow reaction to continue, when still internal pressure is reduced to 0.5MPa, stop reaction, reclaim unreacted tetrafluoroethylene monomer. Release the material and transfer it to a glass flask, separate the solid and li...
Embodiment 3
[0038] After cleaning and drying the reactor, vacuumize and replace with nitrogen until the water content is below 100ppm and the oxygen content is below 10ppm. Vacuumize and fill the tetrafluoroethylene monomer to 0.1MPa, then vacuumize to 0.0001MPa, and then fill 134g monomer CF 2 = CFOCF 2 CF(CF 3 )OCF 2 CF 2 SO 2 F and 255g of monomer C were added to the reaction kettle, the temperature was raised to 100°C, tetrafluoroethylene was introduced until the pressure reached 5MPa, and the addition of tetrafluoroethylene was stopped, and 10mL containing 0.005g perfluorobutyryl compound (CF 3 CF 2 CF 2 CO-OO-CCF 2 CF 2 CF 3 ) and 0.01g cyclohexane initiator and chain transfer agent solution, and continuously add 90g monomer CF 2 = CFOCF 2 CF(CF 3 )OCF 2 CF 2 SO 2 F and 0.5g monomer C, when the pressure in the kettle was reduced to 1MPa, the reaction was stopped, and the unreacted tetrafluoroethylene monomer was recovered. Release the material and transfer it to a gl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com