Pharmaceutical composition for treating glioblastoma and prognostic diagnostic reagent
A technology of glioblastoma and diagnostic reagents, applied in the field of biomedicine, can solve the problems that PDGFRA inhibitors cannot achieve anti-tumor goals, PDGFRA inhibitors cannot effectively anti-tumor, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
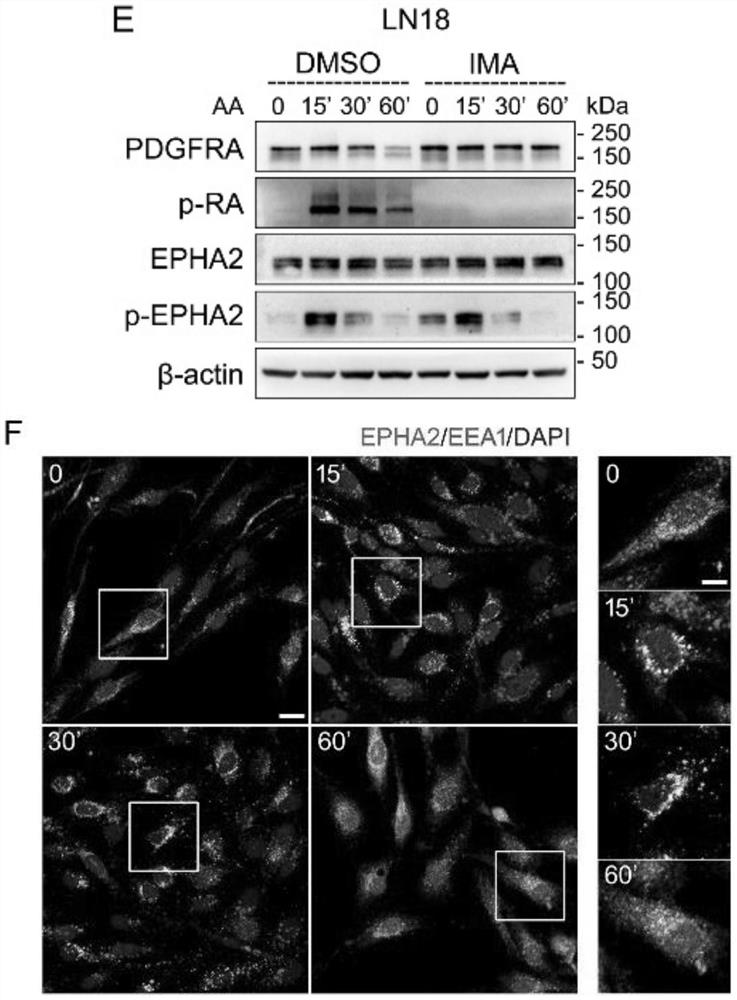
[0044] By western blotting and immunofluorescence imaging experiments, the results are as follows figure 1 It can be concluded that PDGFA can activate EPHA2 independently of PDGFRA in GBM cells.
[0045] 1) Western blotting (Western blot) figure 1 A-E, G, K
[0046]① Extraction of total cell protein: Aspirate the medium, add 5ml of PBS and gently shake to wash the medium, then aspirate and discard the PBS. After adding 1ml of PBS, use a cell scraper to scrape off the cells, and transfer them to a 1.5ml centrifuge tube with a pipette gun. Centrifuge at 800g for 3min at 4°C, discard the supernatant and leave the cell pellet. Add about 10 times volume of RIPA lysate containing PMSF to the cell pellet, and lyse on ice for 30 min. After the lysis was completed, the cell lysate was placed in a centrifuge at 4°C and centrifuged at 25000g for 15min. Aliquot and transfer the centrifuged supernatant to a new 1.5ml centrifuge tube, which is the total cell protein.
[0047] ②Protein...
Embodiment 2
[0066] Through immunohistochemical analysis experiments, the results were as follows figure 2 The clinical significance of EPHA2 and PDGFRA in GBM was drawn.
[0067] 1) Immunohistochemical analysis figure 2 A
[0068] ①Paraffin section: put the paraffin specimen into -20℃ refrigerator for pre-cooling, then slice, spread and air-dry.
[0069] ② Dewaxing: Put the paraffin sections in an oven at 60°C for 30 minutes and then perform dewaxing steps: Xylene I 15 minutes, Xylene II 15 minutes, 100% alcohol for 10 minutes, 95% alcohol for 5 minutes, 85% alcohol for 5 minutes, 75% alcohol 5min, rinse with tap water for 5min, and in PBS for 5min.
[0070] ③Antigen retrieval: Rinse the slices with PBS for 15 minutes. After configuring the repair solution corresponding to the antibody, add an appropriate amount of water to the pressure cooker and heat it to boiling. Put the slices into the pressure cooker for 2min 30s. Then immediately rinse the cooling pressure cooker with tap wa...
Embodiment 3
[0085] Through MTT experiments and mouse model experiments, the results are as follows image 3 It was concluded that the joint inhibition of PDGFRA and EPHA2 expression by IMA and ALW could inhibit GBM cells.
[0086] 1) MTT experiment image 3 A.C
[0087] ① Inoculate an appropriate amount of cells in a 96-well plate, 3000-5000 cells per well, and 100 μL of medium. After the cells adhered to the wall, they were treated with drugs and incubated in a 37°C incubator for 72 hours.
[0088] ② Take out the 96-well plate, discard the old medium, add 100 μL of medium containing MTT solution, continue to incubate at 37°C for 3 hours, then stop the culture, carefully aspirate and discard the medium in the well, add 100 μL DMSO to each well, shake until the crystals are fully dissolved .
[0089] ③Measure the OD value with a microplate reader at 570nm, and record the result.
[0090] 2) Antibody Array image 3 B
[0091] Using the antibody array kit from R&D Systems, scrape the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com